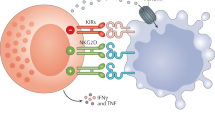
Summary:
Natural killer (NK) cells are important for their ability to recognize and lyse tumor cells and virus infected cells. NK cells express triggering receptors that are specific for non-MHC ligands. This article describes the 35S release cytotoxic assay, which measures the ability of NK cells derived from spleen cells taken from polyIC-treated mice to lyse B-cell leukemia (BCL1) cells. BCL1 cells express ligands for NKp46 on the cell surface membrane and they are sensitive to allogeneic but not syngeneic IL-2 activated natural killer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Slavin S, Weiss L, Morecki S, Weigensberg M . Eradication of murine leukemia with histoincompatible marrow grafts in mice conditioned with total lymphoid irradiation (TLI). Cancer Immunol Immunother 1981; 11: 155–158.
Truitt RL, Shih CY, Lefever AV et al. Characterization of alloimmunization-induced T lymphocytes reactive against AKR leukemia in vitro and correlation with graft-vs-leukemia activity in vivo. J Immunol 1983; 131: 2050–2058.
Weiss L, Weigensberg M, Morecki S et al. Characterization of effector cells of graft vs leukemia (GVL) following allogeneic bone marrow transplantation in mice inoculated with murine B-cell leukemia (BCL1). Cancer Immunol Immunother 1990; 31: 236–242.
Horowitz M, Gale RP, Sondel PM et al. Graft vs leukemia reactions after bone marrow transplantation. Blood 1990; 75: 555–562.
Ruggeri L, Capanni M, Urbani E et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295: 2097–2100.
Rosenberg SA, Lotze MT, Muul LM et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 1987; 316: 889–897.
Cudkowicz G, Bennett M . Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F1 hybrid mice. J Exp Med 1971; 134: 1513–1528.
Rosenberg SA, Lotze MT, Muul LM et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985; 313: 1485–1492.
Cohen P, Vourka-Karussis U, Weiss L, Slavin S . Spontaneous and IL-2 induced anti-leukemic and anti-host effects against tumor-and host-specific alloantigens. J Immunol 1993; 151: 4803–4810.
Ackerstein A, Slavin S, Weiss L, Naparstek E . Immunotherapy in conjunction with autologous bone marrow transplantation. BMT 1990; 5: 38.
Ackerstein A, Kedar E, Slavin S . Use of recombinant human interleukin-2 in conjunction with syngeneic bone marrow transplantation as a model for control of minimal residual disease in malignant hematological disorders. Blood 1991; 78: 1212–1215.
Weiss L, Reich S, Slavin S . Use of recombinant human interleukin-2 in conjunction with bone marrow transplantation as a model for control of minimal residual disease in malignant hematological disorders. I. Treatment of murine leukemia in conjunction with allogeneic bone marrow transplantation and IL2-activated cell-mediated immunotherapy. Cancer Invest 1992; 10: 19–26.
Slavin S, Ackerstein A, Weiss L et al. Immunotherapy of minimal residual disease by immunocompetent lymphocytes and their activation by cytokines. Cancer Invest 1992; 10: 221–227.
Weiss L, Lubin I, Factorowich I et al. Effective graft vs leukemia effects independent of graft vs host disease after T-cell depleted allogeneic bone marrow transplantation in a murine model of B cell leukemia/lymphoma. Role of cell therapy and rIL-2. J Immunol 1994; 153: 2562–2567.
Vourka-Karussis U, Ackerstein A, Pugatsch T, Slavin S . Allogeneic cell-mediated immunotherapy for eradication of MRD: comparison of T-cell and lymphokine activated killer (LAK) cell mediated adoptive immunotherapy in murine models. Exp Hematol 1999; 27: 461–469.
Weiss L, Reich S, Slavin S . The role of antibodies to IL-2 receptor and anti-Asialo GMI antibodies on GVL effects induced by BMT in murine B cell leukemia. Bone Marrow Transplant 1995; 16: 457–461.
Slavin S, Strober S . Spontaneous murine B-cell leukemia. Nature 1978; 272: 624–626.
Katz G, Markel G, Mizrahi S et al. Recognition of HLA-Cw4 but not HLA-Cw6 by the NK cell receptor killer cell Ig-like receptor two-domain short tail number 4. J Immunol 2001; 166: 7260–7267.
Mandelboim O, Lieberman N, Lev M et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 2001; 409: 1055–1060.
Porgador A, Mandelboim O, Restifo NP, Strominger JL . Natural killer cell lines kill autologous beta2-microglobulin-deficient melanoma cells: implications for cancer immunotherapy. Proc Natl Acad Sci USA 1997; 94: 13140–13145.
Moretta L, Biassoni R, Bottino C et al. Surface receptors that regulate the NK cell function: beyond the NK cell scope. Curr Top Microbiol Immunol 2002; 266: 11–22.
Vitetta ES, Yuan D, Krollick K et al. Characterization of the spontaneous murine B cell leukemia (BCL1). III. Evidence for monoclonality using an anti-idiotype antibody. J Immunol 1979; 122: 1649–1654.
Yuan D, Uhr JW, Knapp MR et al. Structural differences between μ chain of cell associated and secreted immunoglobulin. In: Cooper M, Mosier D, Scher I, Vitetta E (eds.). B Lymphocytes in the Immune Response. Elsevier: Amsterdam, 1979; pp 23–31.
Knapp MR, Jones PP, Black SJ et al. Characterization of a spontaneous murine B cell leukemia (BCL1). 1. Cell surface expression of IgM, IgD, Ia and FcR. J Immunol 1979; 123: 992–999.
Weiss L, Morecki S, Vitetta ES, Slavin S . Suppression and elimination of BCL1 leukemia by allogeneic bone marrow transplantation. J Immunol 1983; 130: 2452–2455.
Prigozhina T, Gurevitch O, Morecki S et al. Non-myeloablative allogeneic bone marrow transplantation as immunotherapy for hematologic malignancies and metastatic solid tumors in pre-clinical models. Exp Hematol 2002; 30: 89–96.
Slavin S, Naparstek E, Nagler A et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse post allogeneic bone marrow transplantation. Blood 1996; 87: 2195–2204.
Leshem B, Vourka Karussis U, Slavin S . Correlation between enhancement of graft vs leukemia effects following allogeneic bone marrow transplantation by rIL-2 and increased frequency of cytotoxic T-lymphocyte precursors in murine myeloid leukemia. Cytokines, Cell Mol Ther 2000; 6: 141–147.
Weiss L, Reich S, Slavin S . The role of antibodies to IL-2 receptor and anti-Asialo GMI antibodies on GVL effects induced by BMT in murine B cell leukemia. Bone Marrow Transplant 1995; 16: 457–461.
Moretta L, Bottino C, Pende D et al. Human natural killer cells: their origin, receptors and function. Eur J Immunol 2002; 32: 1205–1211, (Review).
Acknowledgements
We thank the Danny Cunniff Leukemia Research Laboratory; the Gabrielle Rich Leukemia Research Foundation; the Cancer Treatment Research Foundation; the Novotny Trust and the Fig Tree Foundation for their continuous support of our ongoing basic and clinical research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weiss, L., Reich, S., Mandelboim, O. et al. Murine B-cell leukemia lymphoma (BCL1) cells as a target for NK cell-mediated immunotherapy. Bone Marrow Transplant 33, 1137–1141 (2004). https://doi.org/10.1038/sj.bmt.1704475
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704475
Keywords
This article is cited by
-
Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1
Nature Immunology (2006)
-
Autologous control of a highly malignant syngeneic CRNK-16 leukemia in the rat: a role for NK cells
Cancer Immunology, Immunotherapy (2006)