Summary:
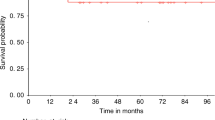
Nonmyeloblative stem cell transplantation (NST, SCT) aims to induce host-versus-graft tolerance for subsequent immunotherapy of underlying disease with alloreactive donor lymphocytes, focusing on well-tolerated conditioning suitable for elderly individuals or for other risk factors. However, there is a subset of high-risk patients who cannot tolerate NST. A new protocol consisting of fludarabine 30 mg/m2 × 6 days (days −8 to –2), very-low-dose busulfan (2 mg/kg × 2 days, days −6 to –5), without anti thymocyte globulin (ATG), was employed in 11 high-risk patients aged 26–58 years. Graft-versus-host-disease (GVHD) prophylaxis consisted of low-dose and short-course cyclosporine-A (CSA) alone. One patient died during the nadir due to pulmonary complications. Other patients showed rapid three-lineage engraftment, without complete aplasia; 6/10 patients did not require platelet transfusion and 8/10 had full donor chimerism without transient mixed chimerism. Owing to intentional selection of highly poor-risk patients, overall mortality was high and only one patient survived. Acute GVHD (⩾grade I) occurred in 8/10 evaluable patients, 5/8 while off CSA; 5/8 developed grade III–IV acute GVHD. It appears that our modified, minimally ablative stem cell transplantation (MST) may be used for high-risk patients in need of allo-SCT. Furthermore, although the MST conditioning is not myeloablative, it results in myeloablation of the host hematopoietic system, mediated by alloreactive lymphocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Slavin S, Nagler A, Naparstek E et al. Non-myeloablative transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and non-malignant hematologic diseases. Blood 1998; 91: 756–763.
Slavin S . Immunotherapy of cancer with alloreactive lymphocytes. Lancet Oncol 2001; 2: 491–498 (Review).
Champlin R, Khouri I, Kornblau S et al. Reinventing bone marrow transplantation. Non-myeloablative preparative regimens and induction of graft-vs-malignancy effect. Oncology 1999; 13: 621–628.
Slavin S, Naparstek E, Nagler A et al. Allogeneic cell therapy for relapsed leukemia following bone marrow transplantation with donor peripheral blood lymphocytes. Exp Hematol 1995; 23: 1553–1562.
Slavin S, Naparstek E, Nagler A et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse post allogeneic bone marrow transplantation. Blood 1996; 87: 2195–2204.
Glucksberg H, Storb R, Fefer A et al. Clinical manifestations of graft-vs-host disease in human recipients of marrow from HLA-matched-sibling donors. Transplantation 1974; 18: 295–304.
Pugatsch T, Oppenheim A, Slavin S . Improved single-step PCR assay for sex identification post-allogeneic sex-mismatched BMT. Bone Marrow Transplant 1996; 17: 273–275.
Nakamura Y, Leppert O, O'Connel P et al. Variable number of tandem repeats (VNTR) markets for human gene mapping. Science 1987; 235: 1616–1622.
Giralt S, Estey E, Albitar M et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-vs-leukemia without myeloablative therapy. Blood 1997; 89: 4531–4536.
Kapelushnik J, Or R, Slavin S, Nagler A . A fludarabine based protocol for bone marrow transplantation in Fanconi's anemia. Bone Marrow Transplant 1997; 20: 1109–1110.
Khouri IF, Keating M, Korbling M et al. Transplant-lite: induction of graft-vs-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 1998; 16: 2817–2824.
McSweeney PA, Niederwieser D, Shizuru JA et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Carella AM, Cavaliere M, Lerma E et al. Autografting followed by non-myeloablative immunosuppressive chemo-therapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin's disease and non-Hodgkin's lymphoma. J Clin Oncol 2000; 18: 3918–3924.
Storb R . Non-myeloablative preparative regimens: how relevant for acute myelogenous leukemia? Leukemia 2001; 15: 662–663.
Clift RA, Buckner CD, Appelbaum FR et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood 1991; 77: 1660–1665.
Alyea E, Neuberg D, Mauch P et al. Effect of total body irradiation dose escalation on outcome following T-cell-depleted allogeneic bone marrow transplantation. Biol Blood Marrow Transplant 2002; 8: 139–144.
Goldman JM, Gale RP, Horowitz MM et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase: Increased risk for relapse associated with T-cell depletion. Ann Intern Med 1988; 108: 806–814.
Weiden PL, Fluornoy N, Sanders JE et al. Antileukemic effect of graft-versus-host disease contributes to improved survival after allogeneic marrow transplantation. Transplant Proc 1981; 13: 248–251.
Weiden PL, Sullivan KM, Fluornoy N et al. Antileukemic effect of chronic graft-vs-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med 1981; 304: 1529–1533.
Horowitz MM, Gale RP, Sondel PM et al. Graft-vs-leukemia reactions after bone marrow transplantation. Blood 1990; 75: 555–562.
Weiss L, Reich S, Slavin S . Use of recombinant human interleukin-2 in conjunction with bone marrow transplantation as a model for control of minimal residual disease in malignant hematological disorders. I. Treatment of murine leukemia in conjunction with allogeneic bone marrow transplantation and IL2-activated cell-mediated immunotherapy. Cancer Invest 1992; 10: 19–26.
Vourka-Karussis U, Karussis D, Ackerstein A, Slavin S . Enhancement of graft versus leukemia effect (GVL) with recombinant human interleukin 2 (rIL-2) following bone marrow transplantation in a murine model for acute myeloid leukemia in SJL/J mice. Exp Hematol 1995; 23: 196–201.
Ji YH, Weiss L, Zeira M et al. Allogeneic cell-mediated immunotherapy of leukemia with immune donor lymphocytes to up-regulate anti-tumor effects and down-regulate anti-host responses. Bone Marrow Transplant, in press.
Morecki A, Levi S, Puyesky Y, Slavin S . Induction of tumor immunity by intact irradiated leukemic B cells (BCL1) bearing a tumor-associated cell surface idiotype and the costimulatory B7 molecule. Cancer Immunol Immunother 1995; 41: 236–242.
Morecki S, Yacovlev E, Gelfand Y et al. Cell therapy with pre-immunized effector cells mismatched for minor histocompatible antigens, in the treatment of a murine mammary carcinoma. J Immunother 2001; 24: 114–121.
Slavin S, Ackerstein A, Morecki S et al. Immunotherapy of relapsed resistant chronic myelogenous leukemia post allogeneic bone marrow transplantation with alloantigens pulsed donor lymphocytes. Bone Marrow Transplant 2001; 28: 795–798.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shapira, M., Or, R., Resnick, I. et al. A new minimally ablative stem cell transplantation procedure in high-risk patients not eligible for nonmyeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant 32, 557–561 (2003). https://doi.org/10.1038/sj.bmt.1704190
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704190