Summary:
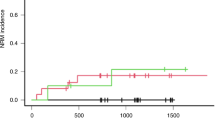
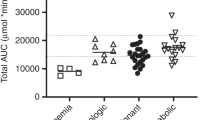
Busulfan is currently used as a main component in the conditioning regimen prior to allogeneic stem cell transplantation (SCT). Several studies have shown a correlation between exposure to busulfan and transplantation-related liver toxicity, such as venoocclusive disease (VOD) in patients undergoing SCT. Busulfan is metabolized mainly through glutathione (GSH). During high-dose therapy, busulfan may deplete hepatocellular levels of GSH. As part of the conditioning therapy, busulfan is usually followed by high doses of cyclophosphamide. The activation of cyclophosphamide yields a cytotoxic metabolite, 4-hydroxy cyclophosphamide, which is highly reactive and detoxified through GSH. According to recent studies using cell lines and animal models N-acetyl-L-cysteine (NAC), a GSH precursor, does not hamper the myeloablative effect of busulfan during conditioning. In the present study, we administered NAC during conditioning to 10 patients at risk of VOD due to pretransplant liver disorders or elevated liver enzymes. No side effects related to the NAC infusions were observed and busulfan concentrations were not affected. All patients became pancytopenic and engrafted with 100% donor cells. None of the patients developed VOD or liver failure. Increased liver enzymes during conditioning decreased or normalized in all patients. We suggest that NAC therapy is safe and does not impair the myeloablative effect of busulfan during conditioning prior to SCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fried W, Kede A, Barone J . Effects of cyclophosphamide and busulfan on spleen-colony-forming units and on hematopoietic stroma. Cancer Res 1977; 37: 1205.
Elson LA . Hematological effects of the alkylating agents. Ann NY Acad Sci 1958; 68: 826.
Santos WG, Tutschka PJ, Brookmeyer R et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med 1983; 309: 1347–1353.
Tutschka PJ, Copelan EA, Klein JP . Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood 1987; 70: 1382–1388.
Ringdén O, Ruutu T, Remberger M et al. A randomised trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: A report from the Nordic Bone Marrow Transplant Group. Blood 1994; 83: 2723–2730.
Grochow LB, Jones RJ, Brundrett RB et al. Pharmacokinetics of busulfan: correlation with venoocclusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61.
Bearman SI . The syndrome of hepatic venoocclusive disease after marrow transplantation. Blood 1995; 85: 3005.
Shulman HM, Hinterberger W . Hepatic venoocclusive disease – liver toxicity syndrome after bone marrow transplantation. Transplantation 1992; 10: 197.
Carreras E, Granena A, Rozman C . Hepatic venoocclusive disease after bone marrow transplant. Blood Rev 1993; 7: 43.
McDonald GB, Sharma P, Matthews DE et al. Venoocclusive disease of the liver after bone marrow transplantation; diagnosis, incidence and predisposing factors. Hepatology 1984; 4: 116.
Jones RJ, Lee KSK, Beschorner WE et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778.
Allen JR, Carstens LA, Katagiri GJ . Hepatic veins of monkeys with venoocclusive disease. Arch Pathol 1969; 87: 279.
Shulman HM, Gown Am, Nugent DJ . Hepatic venoocclusive disease after bone marrow transplantation. Am J Pathol 1987; 127: 549.
Carreras E . Venoocclusive disease of the liver after hematopoietic cell transplantation. Eur J Haematol 2000; 64: 281–291.
Marchand DH, Remmel RP, Abdel-Monem MM . Biliary excretion of a glutathione conjugate of busulfan and 1,4 diiodobutane in the rat. Drug Metab Dispos 1988; 16: 85–92.
Hassan M, Ehrsson H . Metabolism of 14C-busulfan in isolated perfused rat liver. Eur J Drug Metab Pharmacokinet 1987; 12: 71–76.
Ritter CA, Bohnenstengel F, Hofmann Um Kroemer HK, Sperker B . Determination of tetrahydrothiophene formation as a probe of in vitro busulfan metabolism by human glutathione S-transferase A1-1: use of a highly sensitive gas chromatographic–mass spectrometric method. J Chromatogr B 1999; 730: 25–31.
Czerwinski M, Gibbs JP, Slattery JT . Busulfan conjugation by glutathione S-transferase alpha my, and pi. Drug Metab Dispos 1996; 24: 1015–1019.
Gibbs JP, Czerwinski M, Slattery JT . Busulfan-glutathione conjugation catalyzed by human liver cytosolic glutathione S-transferases. Cancer Res 1996; 56: 3678–3681.
De Leve LD, Wang X . Role of oxidative stress and glutathione in busulfan toxicity in cultured murine hepatocytes. Pharmacology 200; 60: 143–154.
Meister A . Glutathione metabolism and its selective modification. J Biol Chem 1988; 263: 17205–17208.
Chyka PA, Butler AY, Holliman BJ, Herman MI . Utility of acetylcysteine in treating poisonings and adverse drug reactions. Drug Saf 2000; 22: 123–148.
Ringden O, Remberger M, Lehmann S et al. N-acetylcysteine for hepatic veno-occlusive disease after allogeneic stem cell transplantation. Bone Marrow Transplant 2000; 25: 993–996.
Czerwinski M, Kiem HP, Slattery JT . Human CD34+ cells do not express glutathione S-transferases alpha. Gene Ther 1997; 4: 268–270.
Wang L, Groves MJ, Hepburn MD, Bowen DT . Glutathione S-transferase enzyme expression in hematopoietic cell lines implies a different protective role for T1 and A1 isoenzymes in erythroid and for M1 in lymphoid lineages. Haematologica 2000; 85: 573–579.
Hassan Z, Hellström-Lindberg E, Alsadi S et al. The effect of modulation of glutathione cellular content on busulfan induced toxicity on hematopoietic cells in vitro and in vivo. Bone Marrow Transplant 2002; 30: 141–147.
Hassan Z, Ljungman P, Ringdén O et al. Pharmacokinetics of liposomal busulfan in man. Bone Marrow Transplant 2000; 27: 479–485.
Hassan M, Ehrsson H . Gas chromatographic determination of busulfan in plasma with electron–capture detection. J Chromatogr 1983; 277: 374–380.
Hassan M, Ljungman P, Ringdén O et al. The effect of busulfan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy therapy related toxicity. Bone Marrow Transplant 2000; 25: 915–924.
Hassan M, Fasth A, Gerritsen B et al. Busulfan kinetics and limited sampling model in children with leukemia and inherited disorders. Bone Marrow Transplant 1996; 18: 843–850.
Rindén O, Remberger M, Carlens S et al. Low incidence of acute graft-versus-host disease using unrelated HLA-A, -B and -DR compatible donors and conditioning including T-cell antibodies. Transplantation 1998; 66: 620–625.
Prescott LF, Donovan JW, Jarvie DR, Proudfoot AT . The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol over dosage. Eur J Clin Pharmacol 1989; 37: 501–506.
Rank N, Michel C, Haertel C et al. N-acetyl increases liver blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double-blind study. Crit Care Med 2000; 28: 3799–3806.
Ahola T, Fellman V, Laaksonen R et al. Pharmacokinetics of intravenous N-acetylcysteine in pre-term new-born infants. Eur J Clin Pharmacol 1999; 55: 645–650.
Glucksberg H, Storb R, Fefer A et al. Clinical manifestation of graft-versus-host disease in human recipients of marrow from HLA-A matched sibling donors. Transplantation 1974; 18: 295–304.
Acknowledgements
This project was supported by grants from the Swedish Children Cancer Foundation (PROJ01/059, 1997/073), the Stockholm's Cancer Foundation (PROJ02/119), the Swedish Cancer Society (0070-B99-13XZC), the Swedish Medical Research Council (K2000-06X-05971-20A), the Cancer Society in Stockholm, the
Tobias Foundation, the FRF Foundation and Karolinska Institutet.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sjöö, F., Aschan, J., Barkholt, L. et al. N-acetyl-L-cysteine does not affect the pharmacokinetics or myelosuppressive effect of busulfan during conditioning prior to allogeneic stem cell transplantation. Bone Marrow Transplant 32, 349–354 (2003). https://doi.org/10.1038/sj.bmt.1704143
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704143
Keywords
This article is cited by
-
Prophylactic NAC promoted hematopoietic reconstitution by improving endothelial cells after haploidentical HSCT: a phase 3, open-label randomized trial
BMC Medicine (2022)
-
Review of the Pharmacokinetics and Pharmacodynamics of Intravenous Busulfan in Paediatric Patients
Clinical Pharmacokinetics (2021)
-
The effect of N-acetyl-l-cysteine (NAC) on liver toxicity and clinical outcome after hematopoietic stem cell transplantation
Scientific Reports (2018)
-
Toxicological effects of fludarabine and treosulfan conditioning before allogeneic stem-cell transplantation
International Journal of Hematology (2017)
-
N-acetyl cysteine for prevention of oral mucositis in hematopoietic SCT: a double-blind, randomized, placebo-controlled trial
Bone Marrow Transplantation (2014)