Summary:
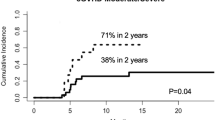
After allogeneic stem cell transplantation (SCT), donor T-cells are primarily responsible for the antihost activity, resulting in graft-versus-host disease (GVHD). Three effector pathways have been described for T-cell cytotoxicity: perforin/granzyme B; Fas/Fas ligand (FasL) and secreted molecules such as TNF-α. The goal of this pilot study was to utilize competitive reverse transcription (RT)-PCR to evaluate the pattern of granzyme B, perforin, FasL and TNF-α gene expression in peripheral blood in patients after SCT. Protein levels of granzyme B, soluble FasL (sFasL) and TNF-α in plasma were also analyzed. Eight patients who underwent allogeneic SCT were included; five were diagnosed with acute GVHD. In the patients diagnosed with acute GVHD, we found increased levels of granzyme B, perforin and FasL mRNA, although this did not correlate with the clinical severity. However, patients with increasing levels of gene expression during acute GVHD treatment may have an increased risk of developing severe acute GVHD, as two out of three patients with increasing immune transcript levels during GVHD therapy developed life-threatening acute GVHD. In conclusion, the quantitative RT-PCR of granzyme B, perforin and FasL may serve as a guide to the clinician in diagnosing acute GVHD and monitoring treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ferrara JL, Deeg HJ . Graft-versus-host disease. N Engl J Med 1991; 324: 667–674.
Ringden O, Deeg HJ . Clinical spectrum of graft-versus-host disease. In: Ferrara JML, Deeg HJ, Burakoff SJ (eds). Graft vs Host Disease. Marcel Dekker, Inc.: New York, 1996, pp 525–529.
Storb R, Thomas ED . Graft-versus-host disease in dog and man: the Seattle experience. Immunol Rev 1985; 88: 215–238.
Hakim FT, Mackall CL . The immune system: effector and target of graft-versus-host disease. In: Ferrara JLM, Deeg HJ, Burakoff SJ (eds). Graft-vs-Host Disease. Marcel Dekker: New York, 1997, pp 257–289.
Korngold R, Sprent J . T-cell subsets in graft-vs.-host disease. In: Burakoff SJ, Deeg HJ, Ferrara JLM, Atkinson K (eds). Graft-vs.-Host Disease. Marcel Dekker: New York, 1990, pp 31–49.
Kagi D, Vignaux F, Ledermann B et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 1994; 265: 528–530.
Lowin B, Hahne M, Mattmann C, Tschopp J . Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 1994; 370: 650–652.
Nagata S, Golstein P . The Fas death factor. Science 1995; 267: 1449–1456.
Berke G . The CTL's kiss of death. Cell 1995; 81: 9–12.
Thomas WD, Hersey P . TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol 1998; 161: 2195–2200.
Via CS, Nguyen P, Shustov A et al. A major role for the Fas pathway in acute graft-versus-host disease. J Immunol 1996; 157: 5387–5393.
Braun MY, Lowin B, French L et al. Cytotoxic T cells deficient in both functional fas ligand and perforin show residual cytolytic activity yet lose their capacity to induce lethal acute graft-versus-host disease. J Exp Med 1996; 183: 657–661.
Graubert TA, DiPersio JF, Russell JH, Ley TJ . Perforin/granzyme-dependent and independent mechanisms are both important for the development of graft-versus-host disease after murine bone marrow transplantation. J Clin Invest 1997; 100: 904–911.
Hattori K, Hirano T, Miyajima H et al. Differential effects of anti-Fas ligand and anti-tumor necrosis factor alpha antibodies on acute graft-versus-host disease pathologies. Blood 1998; 91: 4051–4055.
Lee S, Chong SY, Lee JW et al. Difference in the expression of Fas/Fas-ligand and the lymphocyte subset reconstitution according to the occurrence of acute GVHD. Bone Marrow Transplant 1997; 20: 883–888.
Das H, Imoto S, Murayama T et al. Levels of soluble FasL and FasL gene expression during the development of graft-versus-host disease in DLT-treated patients. Br J Haematol 1999; 104: 795–800.
Glucksberg H, Storb R, Fefer A et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Snover DC . The pathology of acute graft-vs.-host disease. In: Burakoff SJ, Deeg HJ, Ferrara JLM, Atkinson K (eds). Graft-vs.-Host Disease. Marcel Dekker: New York, 1990, pp 337–353.
Holler E, Kolb HJ, Moller A et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood 1990; 75: 1011–1016.
Kanda Y, Tanaka Y, Shirakawa K et al. Increased soluble Fas-ligand in sera of bone marrow transplant recipients with acute graft-versus-host disease. Bone Marrow Transplant 1998; 22: 751–754.
Remberger M, Ringden O, Markling L . TNF alpha levels are increased during bone marrow transplantation conditioning in patients who develop acute GVHD. Bone Marrow Transplant 1995; 15: 99–104.
Ringden O, Remberger M, Runde V et al. Peripheral blood stem cell transplantation from unrelated donors: a comparison with marrow transplantation. Blood 1999; 94: 455–464.
Ringden O, Ruutu T, Remberger M et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood 1994; 83: 2723–2730.
Remberger M, Svahn BM, Hentschke P et al. Effect on cytokine release and graft-versus-host disease of different anti-T cell antibodies during conditioning for unrelated haematopoietic stem cell transplantation. Bone Marrow Transplant 1999; 24: 823–830.
Storb R, Deeg HJ, Whitehead J et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 1986; 314: 729–735.
Remberger M, Storer B, Ringden O, Anasetti C . Association between pretransp.ant thymoglobulin and reduced non-relapse mortality rate after marrow transplantation from unrelated donors. Bone Marrow Transplant 2002; 29: 391–397.
Ringden O, Remberger M, Persson U et al. Similar incidence of graft-versus-host disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings. Bone Marrow Transplant 1995; 15: 619–625.
Ringden O, Remberger M, Carlens S et al. Low incidence of acute graft-versus-host disease, using unrelated HLA-A-, HLA-B-, and HLA-DR-compatible donors and conditioning, including anti-T-cell antibodies. Transplantation 1998; 66: 620–625.
Aschan J . Treatment of moderate to severe acute graft-versus-host disease: a retrospective analysis. Bone Marrow Transplant 1994; 14: 601–607.
Strehlau J, Pavlakis M, Lipman M et al. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc Natl Acad Sci USA 1997; 94: 695–700.
Li B, Hartono C, Ding R et al. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med 2001; 344: 947–954.
Baker MB, Altman NH, Podack ER, Levy RB . The role of cell-mediated cytotoxicity in acute GVHD after MHC-matched allogeneic bone marrow transplantation in mice. J Exp Med 1996; 183: 2645–2656.
Hattori K, Hirano T, Ushiyama C et al. A metalloproteinase inhibitor prevents lethal acute graft-versus-host disease in mice. Blood 1997; 90: 542–548.
Snover DC, Weisdorf SA, Ramsay NK et al. Hepatic graft versus host disease: a study of the predictive value of liver biopsy in diagnosis. Hepatology 1984; 4: 123–130.
Schwaighofer H, Herold M, Schwarz T et al. Serum levels of interleukin 6, interleukin 8, and C-reactive protein after human allogeneic bone marrow transplantation. Transplantation 1994; 58: 430–436.
Remberger M, Ringden O . Serum levels of cytokines after bone marrow transplantation: increased IL-8 levels during severe veno-occlusive disease of the liver. Eur J Haematol 1997; 59: 254–262.
Holler E, Ferrara JLM . Antagonists of inflammatory cytokines: prophylactic and therapeutic applications. In: Ferrara JLM, Deeg HJ, Burakoff SJ (eds). Graft-vs.-Host Disease. Marcel Dekker: New York, 1997, pp 667–692.
Kami M, Matsumura T, Tanaka Y et al. Serum levels of soluble interleukin-2 receptor after bone marrow transplantation: a true marker of acute graft-versus-host disease. Leukemia Lymphoma 2000; 38: 533–540.
Acknowledgements
The authors thank Berit Sundberg for excellent technical assistance, as well as the staff at the Center for Allogeneic Stem Cell Transplantation, Department of Hematology and Pediatrics for competent and compassionate patient care. We also thank Olle Ringdén for critical reading of the manuscript. This study was supported from the following grants: the Swedish Cancer Society (0070-B99-13XAC), the Tore Nilsson foundation for medical research, the Children's Cancer Foundation (1997/073, 2001/012), the Swedish Foundation for Medical Research (SSMF) and the Swedish Medical Society (2001-1299) the Cancer Society in Stockholm, the Tobias Foundation, the FRF Foundation and the Karolinska Institute.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jaksch, M., Uzunel, M., Martinez Cangana, G. et al. Increased levels of immune transcript in patients with acute GVHD after allogeneic stem cell transplantation. Bone Marrow Transplant 31, 183–190 (2003). https://doi.org/10.1038/sj.bmt.1703807
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703807
Keywords
This article is cited by
-
Granzymes A and B serum levels in allo-SCT
Bone Marrow Transplantation (2009)
-
Increase of CCR7− CD45RA+ CD8 T cells (TEMRA) in chronic graft-versus-host disease
Leukemia (2006)
-
Pediatric myelodysplastic syndromes
Current Treatment Options in Oncology (2005)