Abstract
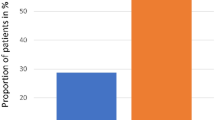
High-dose melphalan (HDM) has been adopted as standard therapy in the treatment of multiple myeloma. This treatment is associated with non-selective cytotoxicity, causing oral mucositis as the major non-hematological side-effect. Amifostine is a cytoprotector which prevents toxicity induced by anticancer therapy. We prospectively compared two groups of patients who either received (group A, n = 21) or did not receive (group B, n = 20) amifostine (740 mg/m2) before HDM (200 mg/m2) followed by autologous peripheral blood progenitor cell transplantation. The occurrence of severe oral mucositis was significantly decreased in group A in comparison to group B (33% vs 65%, P < 0.05). Six patients in group A required opioid analgesic therapy during a mean period of 4.8 days as compared to eight patients for 6.5 days in group B (P = NS). Delayed vomiting was less frequent in group A (43% vs 70%, P = 0.07) and significantly less severe in group A (grade 2–4) vomiting: two patients vs nine patients, P < 0.02). No difference was observed between the two groups in either hematological toxicity after HDM or in response rate. Grade I emesis was the only immediate side-effect observed after amifostine administration. We conclude that amifostine can reduce mucositis induced by HDM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Alexanian R, Dimopoulos M . Management of multiple myeloma Semin Hematol 1995 32: 20 30
Camba L, Durie B . Multiple myeloma. New treatment options Drugs 1992 44: 170 181
Gregory W, Richards M, Malpas J . Combination chemotherapy versus melphalan and prednisolone in the treatment of multiple myeloma: an overview of published trials J Clin Oncol 1992 10: 334 342
Barlogie B, Hall R, Zander A, Dicke K, Alexanian R . High-dose melphalan with autologous bone marrow transplantation for multiple myeloma Blood 1986 67: 1298 1301
Selby P, McElwain T, Nandi A et al. Multiple myeloma treated with high dose intravenous melphalan Br J Haematol 1987 66: 55 62
Attal M, Harousseau J, Stoppa A et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome New Engl J Med 1996 335: 91 97
Cunningham D, Paz-Ares L, Gore M et al. High-dose melphalan for multiple myeloma: long-term follow-up data J Clin Oncol 1994 12: 764 768
Samuels B, Bitran J . High-dose intravenous melphalan: a review J Clin Oncol 1995 13: 1786 1799
Stiff P . Mucositis associated with stem cell transplantation: current status and innovative approaches to management Bone Marrow Transplant 2001 27: (Suppl. 2) S3 S11
Hensley M, Schuchter L, Lindley C et al. American Society of Clinical Oncology clinical practice guidelines for the use of chemotherapy and radiotherapy protectants J Clin Oncol 1999 17: 3333 3355
Davidson D, Grenan M, Sweeney T . Biological characteristics of some improved radioprotectors. In: Brady L (ed.) Radiation Sensitizers Masson: New York 1980 309 320
Glover D, Glick J, Weiler C et al. WR–2721 protects against the hematologic toxicity of cyclophosphamide: a controlled phase II trial J Clin Oncol 1986 4: 584 588
Turrisi A, Glover D, Hurwitz S et al. Final report of the phase I trial of single-dose WR-2721 (S-2-(3-aminopropyl-amino)ethylphosphorothioic acid) Cancer Treat Rep 1986 70: 1389 1393
Kemp G, Rose P, Lurain J et al. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer J Clin Oncol 1996 14: 2101 2112
Yuhas J, Culo F . Selective inhibition of the nephrotoxicity of cis-dichlorodiammineplatinum(II) by WR-2721 without altering its antitumor properties Cancer Treat Rep 1980 64: 57 64
Treskes M, Boven E, Holwerda U et al. Time dependence of the selective modulation of cisplatin-induced nephrotoxicity by WR2721 in the mouse Cancer Res 1992 52: 2257 2260
Mollman J, Glover D, Hogan W, Furman R . Cisplatin neuropathy. Risk factors, prognosis, and protection by WR-2721 Cancer 1988 61: 2192 2195
Schuchter LM, Glick JH . The current status of WR2721 (amifostine) a chemotherapy and radiation therapy protector in biologic therapy of cancer updates. In: De Vita VT, Hellman S, Rosenberg SA (eds) Cancer: Principles and Practices of Oncology JB Lippincott: Philadelphia 1993 1 9
Green D, Bensely D, Schein P . Preclinical evaluation of WR-151327: an orally active chemotherapy protector Cancer Res 1994 54: 738 741
Glover D, Riley L, Carmichael K et al. Hypocalcemia and inhibition of parathyroid hormone secretion after administration of WR-2721 (a radioprotective and chemoprotective agent) New Engl J Med 1983 309: 1137 1141
List A, Heaton R, Glinsmann-Gibson B, Capizzi R . Amifostine protects primitive hematopoietic progenitors against chemotherapy cytotoxicity Semin Oncol 1996 23: (Suppl. 8) 58 63
Schuchter L . Guidelines for the administration of amifostine Semin Oncol 1996 23: (Suppl. 8) 40 43
Bukowski R . Amifostine (ethyol): dosing, administration and patient management guidelines Eur J Cancer 1996 32A: (Suppl. 4) S46 S49
World Health Organization. Handbook for Reporting Results of Cancer Treatment World Health Organisation: Geneva 1979
Capelli D, Santini G, De Souza C et al. Amifostine can reduce mucosal damage after high-dose melphalan conditioning for peripheral blood progenitor cellautotransplant: a retrospective study Br J Haematol 2000 110: 300 307
Cronin S, Uberti J, Ayash L et al. Use of amifostine as a chemoprotectant during high-dose chemotherapy in autologous peripheral blood stem cell transplantation Bone Marrow Transplant 2000 26: 1247 1249
Hartmann J, von Vangerow A, Fels L et al. A randomized trial of amifostine in patients with high-dose VIC chemotherapy plus autologous blood stem cell transplantation Br J Cancer 2001 84: 313 320
Campilho F, Campos A, Vas C et al. Amifostine (AMI) before high-dose mephalan (HDMel) and autologous hematopoietic stem cell transplantation for patients with multiple myeloma (MM) – a case control analysis Blood 2001 98: 839
van der Vijgh W, Korst A . Amifostine (ethyol): pharmacokinetic and pharmacodynamic effects in vivo Eur J Cancer 1996 32A: (Suppl. 4) S26 S30
Shpall E, Stemmer S, Hami L et al. Amifostine (WR-2721) shortens the engraftment period of 4-hydroperoxycyclophosphamide-purged bone marrow in breast cancer patients receiving high-dose chemotherapy with autologous bone marrow support Blood 1994 83: 3132 3137
Pico J, Avila-Garavito A, Naccache P . Mucositis: its occurrence, consequences, and treatment in the oncology setting Oncologist 1998 3: 446 451
Yuhas J . Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid Cancer Res 1980 40: 1519 1524
Wasserman T, Phillips T, Ross G, Kane L . Differential protection against cytotoxic chemotherapeutic effects on bone marrow CFUs by Wr-2721 Cancer Clin Trials 1981 4: 3 6
Acknowledgements
We wish to thank Joelle Réglioni for the technical assistance provided in this study, and Schering-Plough France for supplying amifostine.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thieblemont, C., Dumontet, C., Saad, H. et al. Amifostine reduces mucosal damage after high-dose melphalan conditioning and autologous peripheral blood progenitor cell transplantation for patients with multiple myeloma. Bone Marrow Transplant 30, 769–775 (2002). https://doi.org/10.1038/sj.bmt.1703757
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703757
Keywords
This article is cited by
-
A real-world accuracy of oral mucositis grading in patients undergoing hematopoietic stem cell transplantation
Supportive Care in Cancer (2022)
-
A randomized study of melphalan 200 mg/m2 vs 280 mg/m2 as a preparative regimen for patients with multiple myeloma undergoing auto-SCT
Bone Marrow Transplantation (2016)
-
N-acetyl cysteine for prevention of oral mucositis in hematopoietic SCT: a double-blind, randomized, placebo-controlled trial
Bone Marrow Transplantation (2014)
-
The efficacy of selenium in prevention of oral mucositis in patients undergoing hematopoietic SCT: a randomized clinical trial
Bone Marrow Transplantation (2013)
-
Systematic review of amifostine for the management of oral mucositis in cancer patients
Supportive Care in Cancer (2013)