Abstract
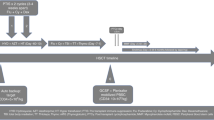
This report concerns a case of long-lasting pure red cell aplasia (PRCA) with a duration of 178 days after major ABO-incompatible allogeneic peripheral blood stem cell transplantation (PBSCT). The patient needed red blood cell transfusion every week from day 54 following PBSCT. He showed no evidence of GVHD and the dose of cyclosporin A (CsA) was reduced rapidly from day 123, followed by the development of chronic GVHD around day 145. The patient no longer needed transfusions from day 167, the reticulocyte count began to increase on day 179, and antidonor isohemagglutinin titers became undetectable. Chronic GVHD induced by tapering of CsA thus appeared to be related to improvement in PRCA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Anasetti C, Amos D, Beatty PG et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma New Engl J Med 1989 320: 197 204
Hershko C, Gale RP, Ho W, Fitchen J . ABH antigens and bone marrow transplantation Br J Haematol 1980 44: 65 73
Sniecinski IJ, Oien L, Petz LD, Blume KG . Immunohematologic consequences of major ABO-mismatched bone marrow transplantation Transplant 1988 45: 530 534
Gmür JP, Burger J, Schaffner A et al. Pure red cell aplasia of long duration complicating major ABO-incompatible bone marrow transplantation Blood 1990 75: 290 295
Worel N, Greinix HT, Schneider B et al. Regeneration of erythropoiesis after related- and unrelated-donor BMT or peripheral blood HPC transplantation: a major ABO mismatch means problems Transplantation 2000 40: 543 550
Takamoto S, Yazawa Y, Yamamoto E et al. ABO incompatible bone marrow transplantation – analysis of 28 cases Vox Sang 1996 70: (Suppl. 2) 139 (Abstr. 3/4B-06 P)
Ohta S, Yokoyama H, Ise T et al. Apheresis therapy for prolonged red cell aplasia after major ABO-mismatched bone marrow transplantation Intern Med 1997 36: 487 491
Heyll A, Aul C, Runde V et al. Treatment of pure red cell aplasia after major ABO-incompatible bone marrow transplantation with recombinant erythropoietin Blood 1991 77: 906 (letter)
Furuya H, Wakayama T, Tanaka J et al. Treatment with bolus methylprednisolone for pure red cell aplasia after ABO incompatible bone marrow transplantation in a patient with chronic myelocytic leukemia Jpn J Clin Hematol 1995 36: 1279 1283
Labar B, Bogdanic V, Nemet D et al. Antilymphocyte globulin for treatment of pure red cell aplasia after major ABO incompatible marrow transplant Bone Marrow Transplant 1992 10: 471 472
Ohashi K, Akiyama H, Takamoto S et al. Treatment of pure red cell aplasia after major ABO-incompatible bone marrow transplantation resistant to erythropoietin Bone Marrow Transplant 1994 13: 335 336
Yang MH, Hsu HC . Pure red cell aplasia after ABO-incompatible allogeneic stem cell transplantation in severe aplastic anemia with response to steroids: a case report and literature review Ann Hematol 2001 80: 299 301
Mielcarek M, Leisenring W, Torok-Storb B, Storb R . Graft-versus-host disease and donor-directed hemagglutinin titers after ABO-mismatched related and unrelated marrow allografts: evidence for a graft-versus-plasma cell effect Blood 2000 96: 1150 1156
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamaguchi, M., Sakai, K., Murata, R. et al. Treatment of pure red cell aplasia after major ABO-incompatible peripheral blood stem cell transplantation by induction of chronic graft-versus-host disease. Bone Marrow Transplant 30, 539–541 (2002). https://doi.org/10.1038/sj.bmt.1703699
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703699
Keywords
This article is cited by
-
Pure red cell aplasia after major or bidirectional ABO incompatible hematopoietic stem cell transplantation: to treat or not to treat, that is the question
Bone Marrow Transplantation (2021)
-
Successful therapy of chronic graft-versus-host disease manifesting as pure red cell aplasia with single-agent rituximab
Bone Marrow Transplantation (2008)
-
The effect of donor leukocyte infusion on refractory pure red blood cell aplasia after allogeneic stem cell transplantation in a patient with myelodysplastic syndrome developing from kostmann syndrome
International Journal of Hematology (2007)
-
Response to high-dose dexamethasone for acquired pure red cell aplasia following ABO-mismatched allogeneic stem cell transplantation
Bone Marrow Transplantation (2006)
-
Pure red cell aplasia after a major ABO-mismatched bone marrow transplant for chronic myeloid leukaemia: response to re-introduction of cyclosporin
Bone Marrow Transplantation (2004)