Abstract
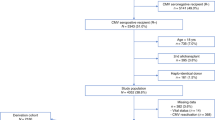
Preemptive antiviral therapy is often employed for CMV prevention following allogeneic BMT. Two common strategies are a screening bronchoscopy for CMV post-BMT or regular CMV antigenemia testing with ganciclovir administration for a positive result. In a randomised trial, we prospectively compared the efficacy of these two preemptive strategies. Consecutive patients were randomised to either a bronchoscopy for CMV on day 35 post BMT or weekly CMV antigenemia testing. If the bronchoscopy was positive for CMV, patients received preemptive ganciclovir for 8–10 weeks. If the antigenemia was positive for CMV, patients received a minimum of 2 weeks of preemptive ganciclovir. The primary endpoint was the development of active CMV disease. One hundred and eighteen allogeneic BMT patients were enrolled (60 in the antigenemia arm and 58 in the bronchoscopy arm). The two groups were comparable with respect to baseline demographic data, underlying disease, conditioning regimen, and immunosuppression. Active CMV disease developed in 7/58 (12.1%) patients in the bronchoscopy arm vs 1/60 patients (1.7%) in the CMV antigenemia arm (P = 0.022). Based on the screening test, 13.8% of patients received preemptive ganciclovir in the bronchoscopy arm vs 48.3% of patients in the antigenemia arm (P < 0.001). There was no significant difference in the rate of graft-versus-host disease, bacteremia, invasive fungal infections or mortality between the two groups. Preemptive therapy based on regular CMV antigenemia monitoring is superior to screening bronchoscopy for the prevention of CMV disease after allogeneic BMT. Bone Marrow Transplantation (2001) 28, 485–490.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Prentice HG, Kho P . Clinical strategies for the management of cytomegalovirus infection and disease in allogeneic bone marrow transplantation Bone Marrow Transplant 1997 19: 135–142
Avery RK, Adal KA, Longworth DL, Bolwell BJ . A survey of allogeneic bone marrow transplant programs in the United States regarding cytomegalovirus prophylaxis and preemptive therapy Bone Marrow Transplant 2000 26: 763–767
Goodrich JM, Bowden RA, Fisher L et al. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant Ann Intern Med 1993 118: 173–178
Winston DJ, Ho WG, Bartoni RN et al. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients Ann Intern Med 1993 118: 179–184
Nguyen Q, Champlin R, Giralt S et al. Late cytomegalovirus pneumonia in adult allogeneic blood and marrow transplant recipients Clin Infect Dis 1999 28: 618–623
Salzberger B, Bowden RA, Fackman RC et al. Neutropenia in allogeneic marrow transplant recipients ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome Blood 1997 90: 2502–2508
Schmidt GM, Horak DA, Niland JC et al. A randomised controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants New Engl J Med 1991 324: 1005–1011
Reddy V, Hao Y, Lipton J et al. Management of allogeneic bone marrow transplant recipients at risk for cytomegalovirus disease using a surveillance bronchoscopy and prolonged preemptive ganciclovir therapy J Clin Virol 1999 13: 149–159
Goodrich JM, Mori M, Gleaves CA et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation New Engl J Med 1991 325: 1601–1607
Boeckh M, Gooley TA, Myerson D et al. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study Blood 1996 88: 4063–4071
Einsele H, Ehninger G, Hebart H et al. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side effects of antiviral therapy after bone marrow transplantation Blood 1995 86: 2815–2820
Ljungman P, Plotkin SA . Workshop on CMV disease. Definitions, clinical severity scores, and new syndromes. Proceedings from the 5th International Cytomegalovirus Conference May 21–24, 1995, Stockholm, Sweden Scan J Infect Dis Suppl 1995 99: 87–89
Van der Bij W, Torensma R, Van Son WJ et al. Rapid immunodiagnosis of active cytomegalovirus infection by monoclonal antibody staining of blood leukocytes J Med Virol 1988 25: 179–188
Mazzulli T, Wood S, Chua R, Walmsley S . Evaluation of the Digene Hybrid Capture System for detection and quantification of human cytomegalovirus viremia in human immunodeficiency virus-infected patients J Clin Microbiol 1996 34: 2959–2962
Li CR, Greenberg PD, Gilbert MJ et al. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis Blood 1994 83: 1971–1979
Humar A, Wood S, Lipton J et al. The clinical utility of CMV surveillance cultures and antigenemia following bone marrow transplantation Bone Marrow Transplant 1999 23: 45–51
Hebart H, Muller C, Loffler J et al. Monitoring of CMV infection: a comparison of PCR from whole blood, plasma-PCR, pp65-antigenemia and virus culture in patients after bone marrow transplantation Bone Marrow Transplant 1996 17: 861–868
Boeckh M, Gallez-Hawkins GM, Myerson D et al. Plasma polymerase chain reaction for cytomegalovirus DNA after allogeneic marrow transplantation: comparison with polymerase chain reaction using peripheral blood leukocytes, pp65 antigenemia, and viral culture Transplantation 1997 64: 108–113
Acknowledgements
Informed consent was obtained from all patients for participation in the study. This work was funded by a grant from the Physicians Services Incorporated.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Humar, A., Lipton, J., Welsh, S. et al. A randomised trial comparing cytomegalovirus antigenemia assay vs screening bronchoscopy for the early detection and prevention of disease in allogeneic bone marrow and peripheral blood stem cell transplant recipients. Bone Marrow Transplant 28, 485–490 (2001). https://doi.org/10.1038/sj.bmt.1703178
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703178
Keywords
This article is cited by
-
Active CMV disease does not always correlate with viral load detection
Bone Marrow Transplantation (2007)