Abstract
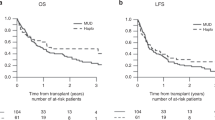
We studied the effect of the CD34+ cell dose on transplant-related mortality (TRM) and survival in 39 patients randomized to receive lenograstim-mobilized PBSCT (n= 20) or BMT (n = 19) from HLA-identical siblings. Both marrow and blood were harvested, and one infused in a double-blind fashion. The median nucleated (7.0 vs 3.2 × 108/kg; P < 0.0001), cd34+ (3.7 vs 1.5 × 106/kg; P = 0.002), CFU-GM (42 vs 19 × 104/kg; P= 0.002), and CD3+ (1.9 vs 0.3 × 108/kg; P < 0.0001) cell doses with pbsct were higher. thirteen patients (6 bmt and 7 pbsct) experienced trm at 15–733 days (median 57); 10 of 20 receiving <2 × 106 CD34+ cells/kg compared with three of 19 receiving ⩾2. Eight of 20 patients receiving <2 × 106 CD34+ cells/kg are alive compared with 14 of 19 receiving ⩾2. In Cox analysis, CD34+ cell dose ⩾2 × 106/kg was associated with lower TRM (RR 0.2, P = 0.01), and higher overall (RR 3.7, P = 0.01) and event-free (RR 3.2, P = 0.02) survival. Other cell populations and the source of stem cells did not affect TRM or survival. We conclude that 2 × 106 CD34+ cells/kg may be the ideal minimum cell dose for allogeneic transplantation although lower doses do not preclude successful therapy. Since the likelihood of obtaining this threshold CD34+ cell number is significantly greater from blood than marrow, PBSCT may be preferable to marrow for allografts from HLA-identical siblings. Bone Marrow Transplantation (2000) 26, 489–496.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Mehta J, Powles R . The future of bone marrow transplantation. In: Atkinson K (ed) Clinical Bone Marrow and Blood Stem Cell Transplantation, 2nd edn Cambridge University Press, Cambridge 2000 1457–1465
Bearman SI, Appelbaum FR, Buckner CD et al. Regimen-related toxicity in patients undergoing bone marrow transplantation J Clin Oncol 1988 6: 1562–1568
Clift RA, Buckner CD, Appelbaum FR et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens Blood 1990 76: 1867–1871
Chao NJ, Forman SJ, Schmidt GM et al. Allogeneic bone marrow transplantation for high-risk acute lymphoblastic leukemia during first complete remission Blood 1991 78: 1923–1927
Copelan EA, Biggs JC, Thompson JM et al. Treatment for acute myelocytic leukemia with allogeneic bone marrow transplantation following preparation with BuCy2 Blood 1991 78: 838–843
Clift RA, Buckner CD, Appelbaum FR et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens Blood 1991 77: 1660–1665
Anderson JE, Appelbaum FR, Fisher LD et al. Allogeneic bone marrow transplantation for 93 patients with myelodysplastic syndrome Blood 1993 82: 677–681
Ringdén O, Ruutu T, Remberger M et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group Blood 1994 83: 2723–2730
Storb R, Etzioni R, Anasetti C et al. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia Blood 1994 84: 941–949
Mehta J, Powles R, Horton C et al. Bone marrow transplantation for primary refractory acute leukaemia Bone Marrow Transplant 1994 14: 415–418
Björkstrand B, Ljungman P, Svensson H et al. Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation Blood 1996 88: 4711–4718
Soiffer RJ, Fairclough D, Robertson M et al. CD6-depleted allogeneic bone marrow transplantation for acute leukemia in first complete remission Blood 1997 89: 3039–3047
Henslee-Downey PJ, Abhyankar SH, Parrish RS et al. Use of partially mismatched related donors extends access to allogeneic marrow transplant Blood 1997 89: 3864–3872
Hjiyannakis P, Mehta J, Milan S et al. Melphalan, single-fraction total-body irradiation and allogeneic bone marrow transplantation for acute leukaemia: review of transplant-related mortality Leuk Lymphoma 1997 25: 565–572
Mehta J, Powles R, Singhal S et al. Autologous bone marrow transplantation for acute myeloid leukemia in first remission: identification of modifiable prognostic factors Bone Marrow Transplant 1995 16: 499–506
Mehta J, Powles R, Treleaven J et al. Long-term follow-up of patients undergoing allogeneic bone marrow transplantation for acute myeloid leukemia in first complete remission after cyclophosphamide-total body irradiation and cyclosporine Bone Marrow Transplant 1996 18: 741–746
Mavroudis D, Read E, Cottler-Fox M et al. CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies Blood 1996 88: 3223–3229
Sierra J, Storer B, Hansen JA et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA-matching, and marrow cell dose Blood 1997 89: 4226–4235
Mehta J, Powles R, Treleaven J et al. Number of nucleated cells infused during allogeneic and autologous bone marrow transplantation: an important modifiable factor influencing outcome Blood 1997 90: 3808–3810
Powles R, Mehta J, Kulkarni S et al. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant disease: a randomised trial Lancet 2000 355: 1231–1237
Singhal S, Powles R, Kulkarni S et al. Comparison of marrow and blood cell yields in a double-blind, randomized study of allogeneic marrow versus blood stem cell transplantation Bone Marrow Transplant 1999 25: 501–505
Mehta J, Powles RL, Mitchell P et al. Graft failure after bone marrow transplantation from unrelated donors using busulphan and cyclophosphamide for conditioning Bone Marrow Transplant 1994 13: 583–587
Mehta J, Powles R, Singhal S et al. Sequential high-dose therapy of adult acute lymphoblastic leukemia: role of maintenance chemotherapy after peripheral blood stem cell transplantation in first remission. In: Dicke KA, Keating A (eds) Autologous Marrow and Blood Transplantation: Proceedings of the Seventh International Symposium. Arlington, Texas. The Cancer Treatment Research and Educational Institute: Arlington 1995 pp 135–144
Singhal S, Powles R, Treleaven J et al. Sequential high-dose therapy of acute lymphoblastic leukemia in adult patients: long-term follow-up Blood 1997 90: (Suppl. 1) 235a (Abstr.)
Dunlop L, Powles R, Singhal S et al. Bone marrow transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia Bone Marrow Transplant 1996 17: 365–369
Singhal S, Powles R, Treleaven J et al. Melphalan-TBI for allogeneic transplantation in acute myeloid leukemia: lack of veno-occlusive disease and hemorrhagic cystitis Blood 1998 92: (Suppl. 1) 658a (Abstr.)
Powles RL, Morgenstern GR, Kay HEM et al. Mismatched family donors for bone marrow transplantation as treatment for acute leukaemia Lancet 1983 1: 612–615
Glucksberg H, Storb R, Fefer A et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors Transplantation 1974 18: 295–304
Mehta J, Kelsey SM, Chu P et al. Amphotericin B lipid complex (ABLC) for the treatment of confirmed or presumed fungal infections in immunocompromised patients with hematologic malignancies Bone Marrow Transplant 1997 20: 39–43
Singhal S, Mehta J, Powles R et al. Three weeks of ganciclovir for cytomegaloviraemia after allogeneic bone marrow transplantation Bone Marrow Transplant 1995 15: 777–781
Mehta J, Powles R, Treleaven J et al. Outcome of acute leukemia relapsing after bone marrow transplantation: utility of second transplants and adoptive immunotherapy Bone Marrow Transplant 1997 19: 709–719
Mehta J, Powles R, Treleaven J et al. Induction of graft-versus-host disease as immunotherapy of leukemia relapsing after allogeneic transplantation: single-center experience of 32 adult patients Bone Marrow Transplant 1997 20: 129–135
Powles R, Singhal S, Treleaven J et al. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery after transplantation Blood 1998 91: 3481–3486
Ketterer N, Salles G, Raba M et al. High CD34(+) cell counts decrease hematologic toxicity of autologous peripheral blood progenitor cell transplantation Blood 1998 91: 3148–3155
Schmitz N, Bacigalupo A, Hasenclever D et al. Allogeneic bone marrow transplantation vs filgrastim-mobilised peripheral blood progenitor cell transplantation in patients with early leukaemia: first results of a randomised multicentre trial of the European Group for Blood and Marrow Transplantation Bone Marrow Transplant 1998 21: 995–1003
Acknowledgements
This study was supported by Chugai Pharma UK, the Bud Flanagan Leukaemia Fund, and the Cancer Research Campaign.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Singhal, S., Powles, R., Treleaven, J. et al. A low CD34+ cell dose results in higher mortality and poorer survival after blood or marrow stem cell transplantation from HLA-identical siblings: should 2 × 106 CD34+ cells/kg be considered the minimum threshold?. Bone Marrow Transplant 26, 489–496 (2000). https://doi.org/10.1038/sj.bmt.1702542
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702542
Keywords
This article is cited by
-
Cryopreserved versus fresh peripheral blood allogeneic stem cell transplantation outcomes in patients receiving post-transplant cyclophosphamide for graft-versus-host prophylaxis during the COVID-19 pandemic: a single center experience
International Journal of Hematology (2023)
-
Bone Marrow Grafts From Pediatric Donors May Contain A Considerable Number of Hematogones
Indian Journal of Hematology and Blood Transfusion (2022)
-
Pluronic-F127/Platelet Microvesicles nanocomplex delivers stem cells in high doses to the bone marrow and confers post-irradiation survival
Scientific Reports (2020)
-
Bone marrow aspiration technique has deteriorated in recent years
Bone Marrow Transplantation (2015)
-
Early engraftment of G-CSF-primed allogeneic bone marrow transplantation in pediatric patients regardless of donor–recipient weight differences
Annals of Hematology (2012)