Abstract
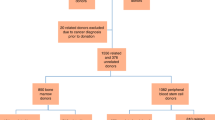
One hundred and one donors who had received filgrastim (rhG-CSF) for the purpose of donating either granulocytes or peripheral blood stem cells (PBSC) for their relatives more than 3 years ago were contacted. All donors had received daily rhG-CSF at a median dose of 16 μg/kg/day (range 3–16) for a median of 6 days (range 3–15 days). All collection procedures were completed and short-term side-effects of rhG-CSF were mild in the majority of the donors. At a median time interval of 43.13 months (range 35–73), the donors were contacted to assess whether adverse effects related to rhG-CSF administration had occurred. Prior to rhG-CSF two donors had cancer, one had a myocardial infarction, one was hepatitis C virus positive, one had a history of sinusitis, one had Graves’ disease and two had arterial hypertension. None worsened with the rhG-CSF administration but the donor with a history of infarction had an episode of angina following apheresis, and the donor with Graves’ disease had a stroke 15 months after rhG-CSF. Two pregnancies occurred after the rhG-CSF administration and one donor was 2–3 weeks pregnant during rhG-CSF treatment. Three pregnancies resulted in two normal births and one in a spontaneous abortion of a pregnancy which occurred more than 2 years following rhG-CSF. In the time following rhG-CSF administration two donors developed cancer (breast and prostate cancer) at a follow-up of 70 and 11 months, respectively. One donor developed lymphadenopathy 38 months after the rhG-CSF, which spontaneously resolved. Blood counts were obtained in 70 donors at a median follow up of 40.4 months (range 16.8–70.8). Hematocrit was 43% (median, range 36.8–48), white blood cells were 5.7 × 109/l (median, range 3–14), granulocytes 3.71 × 109/l (median, range 1.47–10.36), lymphocytes 1.67 × 109/l (median, range 0.90–3.96), monocytes 0.46 × 109/l (median, range 0.07–0.87) and platelet counts were 193.0 × 109/l (median, range 175.0–240.0). This study indicates that short-term administration of rhG-CSF to normal donors for the purpose of mobilizing the PBSC or granulocytes appears safe and without any obvious adverse effects more than 3 years after the donation. Bone Marrow Transplantation (2000) 25, 85–89.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
McCredie KB, Hersh EM, Freireich EJ . Cells capable of colony formation in the peripheral blood of man Science 1971 171: 293–294
Socinski MA, Cannistra SA, Elias A et al. Granulocyte–macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man Lancet 1988 i: 1194–1198
Weaver CH, Buckner CD, Longin K et al. Syngeneic transplantation with peripheral blood mononuclear cells collected after the administration of recombinant human granulocyte colony-stimulating factor Blood 1993 82: 1981–1984
Russell NH, Hunter A, Rogers S et al. Peripheral blood stem cells as an alternative to marrow for allogeneic transplantation Lancet 1993 341: 1482–1483
Bensinger WI, Price TH, Dale DC et al. The effects of daily recombinant human granulocyte colony stimulating factor administration on normal granulocyte donors undergoing leukapheresis Blood 1993 81: 1883–1888
Anderlini P, Przepiorka D, Seong D et al. Clinical toxicity and laboratory effects of granulocyte-colony-stimulating factor (filgrastim) mobilization and blood stem cell apheresis from normal donors, and analysis of charges for the procedures Transfusion 1996 36: 590–595
Majolino I, Cavallero AM, Bacigalupo A et al. Mobilization and collection of PBSC in healthy donors: a retrospective analysis of the Italina bone Marrow Transplantation Group (GITMO) Haematologica 1997 82: 47–52
Sakamaki S, Matsunaga T, Hirayama Y et al. Haematological study of healthy volunteers 5 years after G-CSF (reply letter) Lancet 1995 346: 1432–1433
Miflin G, Charley C, Stainer C et al. Stem cell mobilization in normal donors for allogeneic transplantation: analysis of safety and factors affecting efficacy Br J Haematol 1996 95: 345–348
Stroneck DF, Clay ME, Herr G et al. Blood counts in healthy donors 1 year after the collection of granulocyte-colony-stimulating factor-mobilized progenitor cells and the results of a second mobilization and collection Transfusion 1997 37: 304–308
Bensinger WI, Deeg HJ . Transfusion support and donor considerations in marrow transplantation. In: Sacher RA, AuBuchon JP (eds) Marrow Transplantation: Practical and Technical Aspects of Stem Cell Reconstitution American Association of Blood Banks: Bethesda 1992 pp 157–184
Bortin MM, Buckner CD . Major complications of marrow harvesting for transplantation Exp Hematol 1983 11: 916–921
Buckner CD, Clift RA, Sanders JE et al. Marrow harvesting from normal donors Blood 1984 64: 630–634
Stroncek DF, Holland PV, Bartch G et al. Experiences of the first 493 unrelated marrow donors in the National Marrow Donor Program Blood 1993 81: 1940–1946
Parkkali T, Volin L, Sirèn MK et al. Acute iritis induced by granulocyte colony-stimulating factor used for mobilization in a volunteer unrelated peripheral blood progenitor cell donor (case report) Bone Marrow Transplant 1996 17: 433–434
Huhn RD, Yurkow EJ, Tushinski R et al. Recombinant human interleukin-3 (rhIL-3) enhances the mobilization of peripheral blood progenitor cells by recombinant human granulocyte colony-stimulating factor (rhG-CSF) in normal volunteers Exp Hematol 1996 24: 839–847
Becker PS, Wagle M, Matous S et al. Spontaneous splenic rupture following administration of granulocyte colony-stimulating factor (G-CSF): occurrence in an allogeneic donor of peripheral blood stem cells Biol Blood Marrow Transplant 1997 3: 45–49
Dreger P, Suttorp M, Haferlach T et al. Allogeneic granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for treatment of engraftment failure after bone marrow transplantation Blood 1993 81: 1404–1407
Fossa SD, Poulsen JP, Aaserud A . Alkaline phosphatase and lactate dehydrogenase changes during leucocytosis induced by G-CSF in testicular cancer Lancet 1992 340: 1544
Russell N, Gratwohl A, Schmitz N . The place of blood stem cells in allogeneic transplantation (annotation) Br J Haematol 1996 93: 747–753
Anderlini P, Körbling M, Dale D et al. Allogeneic blood stem cell transplantation: considerations for donors (editorial) Blood 1997 90: 903–908
Medlock ES, Kaplan DL, Cecchini M et al. Granulocyte colony-stimulating factor crosses the placenta and stimulates fetal rat granulopoiesis Blood 1993 81: 916–922
Welte K, Boxer LA . Severe chronic neutropenia: pathophysiology and therapy Semin Hematol 1997 34: 267–278
Falanga A, Marchetti M, Oldani E et al. Changes of meostatic parameters in health donors administered G-CSF for peripheral blood progenitor cell (PBPC) collection Bone Marrow Transplant 1996 17: (Suppl.) S72
Lowenberg B, Touw IP . Hematopoietic growth factors their receptors in acute leukemia (review) Blood 1993 81: 281–292
Avalos BR, Gasson JC, Hedvat C et al. Human granulocyte colony-stimulating factor: biologic activities and receptor characterization on hematopoietic cells and small cell lung cancer cell lines Blood 1990 75: 851–857
Tachibana M, Miyakawa A, Uchida A et al. Granulocyte colony-stimulating factor receptor expression on human transitional cell carcinoma of the bladder Br J Cancer 1997 75: 1489–1496
Heil G, Hoelzer D, Sanz MA et al. A randomized, double-blind, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia Blood 1997 90: 4710–4718
Moore JO, Dodge RK, Amrein PC et al. Granulocyte recovery after intensive postremission chemotherapy for acute myeloid leukemia with aziridinyl benzoquinone and mitoxantrone: Cancer and Leukemia Group B study 9022 Blood 1987 89: 780–788
Freedman MH . Safety of long-term administration of granulocyte colony-stimulating factor for severe chronic neutropenia Curr Opin Hematol 1997 4: 217–224
Ohara A, Kojima S, Hamajima N et al. Myelodysplastic syndrome and acute myelogenous leukemia as a late clonal complication in children with acquired aplastic anemia Blood 1997 90: 1009–1013
Parker SL, Tong T, Bolden S et al. Cancer statistics, 1997 CA Cancer J Clin 1997 47: 5–27
Hasle H, Olsen JH . Cancer in relatives of children with myelodysplastic syndrome, acute and chronic myeloid leukaemia Br J Haematol 1997 97: 127–131
Acknowledgements
This work was supported by Grants: CA 18029, CA 47748, CA 18221, CA 15704, CA 09515, the Jose Carreras Foundation Against Leukemia, and the Joseph Steiner Fund.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cavallaro, A., Lilleby, K., Majolino, I. et al. Three to six year follow-up of normal donors who received recombinant human granulocyte colony-stimulating factor. Bone Marrow Transplant 25, 85–89 (2000). https://doi.org/10.1038/sj.bmt.1702072
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702072
Keywords
This article is cited by
-
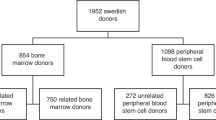
Incidence of cardiovascular disease in healthy Swedish peripheral blood stem cell donors – a nationwide study
Bone Marrow Transplantation (2024)
-
Long-term follow-up of cancer and catastrophic diseases in hematopoietic stem cell donors: a comprehensive matched cohort study
Bone Marrow Transplantation (2024)
-
Molecular investigation of Feline Panleukopenia in an endangered leopard (Panthera pardus) – a case report
BMC Veterinary Research (2023)
-
Cancer incidence in healthy Swedish peripheral blood stem cell donors
Bone Marrow Transplantation (2022)
-
An unusual hematopoietic stem cell transplantation for donor acute lymphoblastic leukemia: a case report
BMC Cancer (2020)