Abstract
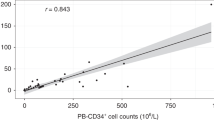
Three different methods for determination of CD34+ cells in G-CSF-mobilized peripheral blood were compared. The methods were: the Milan/Mulhouse protocol, the ISHAGE guidelines for CD34+ cells enumeration and our own protocol. The procedure we have adopted is essentially a Milan/Mulhouse protocol-derived methodology combined with a multiparametric approach using the PAINT-A-GATE software analysis program. The samples were collected from 70 patients affected by acute leukemia, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, myeloma and breast cancer who were scheduled to receive autologous PBSC transplantation. PBSC collection was performed following mobilization with subcutaneous G-CSF at 5–10 μg/kg/day. A minimum target of 2 × 106/kg CD34+ cells was considered an acceptable harvest to ensure a safe transplant. On average, three aphereses per patient were performed and a total of 204 apheresis samples were analyzed. Regression analysis of the percentage and absolute number of CD34+ cells, as calculated with each method, achieved an excellent correlation in spite of methodological differences. In fact, both CD34+dim and CD34+CD45− events were included in our gating strategy. In the setting of a triple staining associating CD34, CD38 and CD45, we identified a variable fraction of CD34+CD38+CD45− cells which would be otherwise undetected due to its CD45 negativity. To this end, we used a new technology referred to as laser-scanning cytometry (LSC) which allowed the isolation and morphological identification of CD34+CD45− cells. By comparing CD34+CD45+ and CD34+CD45− cells, we found that they share a common morphology, thus confirming the hypothesis that the latter are to be considered for CD34+ cell calculation. The median number of CD34+ cells/kg, as calculated by the three methods, was: 4.79 × 106/kg (range 1–570) for the Milan/Mulhouse protocol, 3.9 × 106/kg (range 0.8–498) for the ISHAGE one, and 5.17 × 106/kg (range 2–599) for our protocol. The median time to ANC and PLT engraftment was 11 (range 9–24) and 20 (range 10–70) days, respectively. Our protocol achieved the best correlation between CD34+ cells/kg and time to ANC/PLT recovery according to the Spearman’s rank test (r = −40 and P < 0.015 for anc, r= −46 and P = 0.005 for PLT). We conclude that (1) CD45 does not appear the ideal partner of HPCA-2 for determination of hematopoietic progenitors in mobilized peripheral blood; and (2) for clinical application, a single staining with 8G12 appears simple, reliable and feasible when rigorous procedures for sample preparation and acquisition are followed and an adequate software for multiparametric analysis is available.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Venditti, A., Battaglia, A., Del Poeta, G. et al. Enumeration of CD34+ hematopoietic progenitor cells for clinical transplantation: comparison of three different methods. Bone Marrow Transplant 24, 1019–1027 (1999). https://doi.org/10.1038/sj.bmt.1702013
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702013