Abstract
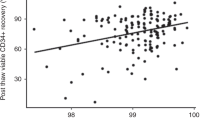
CD34+ cells were purified in bulk from apheresis-collected cells of children with cancer using monoclonal antibody (MoAb) and magnetic beads (Baxter ISOLEX system). To improve the purity of the final product for possibly better tumor cell purging and to make the manufacturer’s original procedure more cost-effective, we incubated the cells for 30 min with l-phenylalanine methylester hydrochloride (PME) to reduce the cell number by removing contaminating granulocytes and monocytes in the initial step before incubation with MoAb. Our modification prevented nonspecific interactions between MoAb and magnetic beads, and thereby saved expensive materials for purification. A total of 40 purifications were performed with samples containing a mean of 3.1 × 109 blood cells mobilized from 15 children by chemotherapy plus granulocyte colony-stimulating factor (G-CSF). The entire purification procedure, from the end of apheresis to storage, was completed within 5 h. After incubation with PME and double-layered (40/60%) Percoll separation, the number of CD34+ cells was reduced to 48 ± 29%, which suggests the possibility that half of the CD34+ cells in the inoculum were nonclonogenic in the hematopoietic progenitor assay. PME/Percoll-treated cells were then subjected to a final isolation procedure with MoAb according to the manufacturer’s suggestions, and 52 ± 42% and 32 ± 22%, respectively, of the CFU-GM and CD34+ cells present in the initial bag inoculums were recovered. The recovery rates were, respectively, 54% and 67%, when the calculation was limited to the isolation procedure with MoAb. The purity of isolated CD34+ cells and the plating efficiency in methylcellulose culture were, respectively, 77 ± 24% and 33 ± 13%. Fourteen children were subsequently autografted with purified CD34+ cells after marrow ablative chemotherapy. The median number of days to achieve an ANC of 0.5 × 109/l was 12 and that to achieve a platelet count of 50 × 109/l was 22.5, which were comparable to those in our historical group of 55 patients who underwent transplant with unmanipulated blood cells (13 and 16 days). These results suggest that our modified purification procedure with PME is useful for the initial reduction of cell numbers to save costly materials, and that cells isolated by this procedure can be directly used in clinical transplantation procedures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawano, Y., Takaue, Y., Law, P. et al. Clinically applicable bulk isolation of blood CD34+ cells for autografting in children. Bone Marrow Transplant 22, 1011–1017 (1998). https://doi.org/10.1038/sj.bmt.1701479
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1701479
Keywords
This article is cited by
-
Large-scale mobilization and isolation of CD34+ cells from normal donors
Bone Marrow Transplantation (2000)
-
Using peripheral blood progenitor cells (PBPC) for transplantation in pediatric patients: a state-of-the-art review
Bone Marrow Transplantation (2000)