Abstract
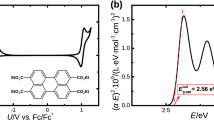
I am grateful to the Editors for affording me the opportunity of writing a final letter in this discussion. The fundamental idea underlying my earlier letter1 was that the carbon atoms in cyclopropane and ethylene oxide were in a hybridization state close to that of the carbon atoms in ethylene. The evidence was then strong. Dr. J. W. Linnett has since pointed out that the CH stretching force-constant in these molecules is much closer to the ethylene than to the paraffinic CH2 value. This developed a point already made, that the height of the strong Raman frequencies indicated the same conclusion. The Bastiansen–Hassel determination of the angle HCH in C3H8 as 118° ± 2 provides further strong evidence for my fundamental point. (The smaller angle in the 1,1 dichloro derivative (112° ± 4 2) is expected since the angle ClCCl in the comparable 1,1 dichloro ethylene is reported as several degrees smaller than the angle HCH in ethylene.) Dr. H. A. Skinner's finding of accord between the Bastiansen–Hassel model and the measured moments of inertia reinforces the argument. Evidence from widely separated sources all fits together and shows that ethylene oxide and cyclopropane can be regarded as formed from CH2 groups the carbon atoms of which are in a state of hybridization close to that of ethylenic carbon atoms. It is this most significant conclusion that a successful theory of these molecules has to explain and the implications of which have to be drawn out.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Walsh, Nature, 159, 712 (1947).
O'Gorman and Schomaker, J. Amer. Chem. Soc. 68, 1138 (1946).
Robinson, Nature, 160, 162 (1947).
Bateman, Nature, 160, 56 (1947).
Sugden, Nature, 160, 367 (1947).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WALSH, A. Letter. Nature 160, 902–903 (1947). https://doi.org/10.1038/160902b0
Issue Date:
DOI: https://doi.org/10.1038/160902b0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.