Abstract
Prepulse inhibition (PPI) of the acoustic startle response (ASR) is a form of sensorimotor gating, defined as an inhibition of the startle response when a low intensity stimulus, the prepulse, precedes the startling stimulus. Deficits in PPI have been reported in schizophrenia and other psychiatric/neurological disorders, and correlate with symptom severity in schizophrenia, suggesting that deficient PPI per se or abnormalities in neural circuits regulating PPI may cause some symptoms of schizophrenia. If so, then genes conferring reduced PPI may contribute toward genetic vulnerability to schizophrenia. Studies with selectively bred rodent strains indicate that PPI is under genetic control; however, the identity of the relevant genes is unknown. The current study used recombinant congenic mouse strains derived from C57BL/6J and A/J parents to assess genetic variability in PPI and in ASR and to identify provisional quantitative trait loci (QTLs) modulating these phenotypes. Significant between-strain differences in ASR and in PPI at each of several prepulse intensities (75, 80, 85, 90, 95 dB) were found. Correlations between PPI at the various prepulse intensities were highly significant, suggesting appreciable overlap in genetic regulation of PPI across prepulse intensities. Five QTLs (chromosomes 3, 5, 7, 16) associated with PPI across all prepulse intensities, but not with ASR, were identified. Two additional QTLs (chromosomes 2, 11) associated with both PPI and ASR were found. Fifteen QTLs were associated with ASR alone. Data on genotypes of informative congenic strains were used to support probable involvement of loci modulating PPI and to narrow the probable chromosomal location of QTLs. If confirmed, these QTLs may suggest candidate genes directing novel mechanisms for regulation of PPI
Similar content being viewed by others
Main
There is strong evidence that several genes with additive or interactive effects, together with environmental factors, contribute to confer susceptibility to schizophrenia (Carlson and Willott 1996). Given this complexity, one approach to uncover genetic susceptibility factors in schizophrenia has been to search for genes regulating specific endophenotypes within schizophrenia. Prepulse inhibition (PPI) of the startle response is a candidate phenotype which may be relevant to such a strategy.
The acoustic startle response (ASR) consists of a strong activation of antagonistic muscle groups throughout the body in response to a sudden, relatively intense acoustic stimulus. Prepulse inhibition refers to an inhibition of the startle response when a low intensity stimulus, the prepulse, precedes the startling stimulus. PPI is a form of sensorimotor gating that is conserved across species, and can be measured under nearly identical conditions in humans and experimental animals (Swerdlow et al. 1999).
Deficits in PPI in schizophrenic compared with control subjects have been reproducibly observed in numerous studies (Braff et al. 1978, 1999; Grillon et al. 1992; Cadenhead et al. 2000; Kumari et al. 2000; Parwani et al. 2000; Swerdlow and Geyer 1998). Importantly, decreased PPI appears to be a familial trait and not a secondary consequence of the gross behavioral impairment accompanying schizophrenia, as PPI deficits are also observed in non-affected relatives of schizophrenic subjects and in non-medicated persons with schizotypal personality disorder (Cadenhead et al. 1993, 2000). In schizophrenia, PPI deficits have been reported to correlate with core cognitive symptoms such as thought disorder (Perry and Braff 1994; Perry et al. 1999), with abnormalities on neuropsychological testing (Butler et al. 1992) and with clinical measures of illness severity (Braff et al. 1999; Light and Braff, 1999; Weike et al. 2000). Thus it has been hypothesized that some symptoms of schizophrenia related to information processing may be caused by abnormalities either in PPI or in the neural circuits that regulate PPI (Swerdlow and Geyer 1998). This idea is strengthened by the fact that brain regions known to modulate PPI (e.g. hippocampus, prefrontal cortex, nucleus accumbens/striatum, globus pallidus, thalamus) are also areas reported to be affected in schizophrenia and other disorders characterized by reduced PPI (Swerdlow and Geyer 1998; Swerdlow et al. 1999). As well, neurotransmitter systems exerting important modulation of PPI (e.g. dopamine, glutamate, serotonin) can also modulate psychotic symptoms (Swerdlow and Geyer 1998). If, as is postulated, deficits in the same neural circuitry contribute to decreased PPI and symptoms of schizophrenia, then genes conferring reduced PPI may contribute toward a constitutional vulnerability to the disorder.
Although most repeatedly reported in schizophrenia, deficits in PPI have been reported in other disorders such as obsessive compulsive disorder (Swerdlow et al. 1993, 1994), Tourette's syndrome (Swerdlow et al. 2001; Castellanos et al. 1996) and Huntington's disease (Swerdlow et al. 1995) suggesting that PPI deficits may play a role in several disorders with abnormalities in shared components of the specific neural circuitry regulating PPI (Swerdlow and Geyer 1998). It is therefore possible that the identification of genes involved in the modulation of PPI may also be relevant to these disorders.
Previous studies on selectively bred, inbred, and recombinant inbred mouse and rat strains have demonstrated wide strain variation in startle responses to both acoustic and tactile stimuli and PPI of these responses Marks et al. 1989; Ellenbroek et al. 1995; Bullock et al. 1997; Logue et al. 1997; Paylor and Crawley 1997; McCaughran et al. 1999; Palmer et al. 2000). This variation indicates that ASR and PPI are, in fact, under genetic control. However, genes regulating PPI have not yet been identified. Quantitative trait locus (QTL) analysis attempts to map genes involved in a trait by detecting significant associations between the phenotype of interest and multiple chromosomal markers. The current study used a series of recombinant congenic strains (RCS) of mice to perform such QTL analysis. RCSs were obtained through two rounds of backcrossing of F1 progeny with either of two parental strains (C57BL/6J and A/J), followed by eighteen to thirty rounds of inbreeding, leading to inbred strains in each of which a small proportion (13.25%) of the genome of one parental strain has been introduced on the other parental genome background and vice versa (Fortin et al. 2001). Thus the RCS system transforms a multigenic trait into a series of single gene traits, where each gene contributing to the multigenic control of the phenotype can be mapped and studied separately (Demant and Hart 1986). RCSs have the potential to detect QTLs even if parental strains do not show phenotypic differences, because genes interacting in the parental strain may be separated in the RCSs. In addition, RCSs are advantageous for correlative phenotypic studies because each mouse in a particular strain represents an inbred “permanent” replica of the appropriate chromosomal recombination producing the informative phenotype (Demant and Hart 1986).
The main aims of the current study were: (1) to determine variability in ASR, and in PPI of acoustic startle, among the RCSs generated from C57BL/6J and A/J mice; (2) to determine genetic correlations between PPI at a range of prepulse intensities and between ASR and PPI; and (3) to identify provisional QTLs modulating either ASR or PPI or both.
MATERIALS AND METHODS
Animals
The mice used in this study were provided by Xenon Genetics Research Inc. (Montreal, Quebec) and were born and raised at the animal care facility of the Montreal General Hospital Research Institute. Recombinant congenic strains (RCSs) were generated from A/J and C57BL/6J progenitors by two rounds of backcrossing of F1 progeny with either of the two parental strains, followed by 18–30 rounds of inbreeding to fix allelic combinations. This procedure yielded 15 C57BL/6J → A/J (primarily A/J background, AcB strains heretofore labeled A) and 22 A/J → C57BL/6J (primarily C57BL/6J background, BcA strains heretofore labeled B) RC strains, covering the differences in the two progenitor strains across the entire genome (Fortin et al. 2001). The degree of homozygosity of introduced genes, estimated from genotyping data on the RCSs, is greater than 99%. In the AcB set, approximately 79% of the C57BL/6J genome has been transferred in independent strains, while in the BcA set, approximately 84% of the A/J genome is represented in the various strains with the C57BL/6J background.
Animals were generated using routine breeding practices and were weaned and identified by ear punching at 3 weeks of age. Mice of a single strain and gender were housed together in groups of five per cage, at an ambient temperature of 22 ± 1°C, with lights on at 6 A.M. and off at 6 P.M., and with access to standard mouse chow and water ad libitum.
Only male mice at 9–10 weeks of age were used for behavioral testing. At 8–9 weeks of age mice were transferred from the Montreal General Hospital to the Douglas Hospital Research Center, where behavioral testing took place. Animals were allowed a minimum of five days to acclimatize to the new facility before behavioral testing.
All procedures with animals were performed in accordance with guidelines from the Canadian Council on Animal Care and were approved by the McGill University Animal Care Committee.
Behavioral Testing
Startle reactivity was measured using two SR-LAB startle response chambers (San Diego Instruments, San Diego, CA). Each sound-attenuated and ventilated chamber contained a Plexiglass cylinder of inner diameter 3.7 cm and length 6.7 cm. The cylinder was mounted atop a piezoelectric transducer, which detected vibrations caused by movement of the animal. An SR-LAB calibration unit was used to produce consistent response sensitivity between chambers and across days of testing. Background noise and all acoustic stimuli were generated by an SR-Lab sound generating system and consisted of white noise. Sound intensity within the chambers was calibrated using a Radio Shack digital sound level meter (A scale). A microcomputer control unit digitized and stored startle responses and also controlled timing and presentation of acoustic stimuli. Startle amplitude was defined as the average of 65 readings taken at 1 msec intervals, beginning at stimulus onset.
Startle testing took place between 9 A.M. and 5 P.M. The startle session began with a 5-min acclimatization period in the presence of 70 dB background noise, which continued throughout the session. After this habituation period, the animals were presented with one orienting pulse-alone trial (120 dB (i.e., 50 dB above background) for 30 msec); data from this trial were discarded. Next, six blocks of trials were delivered. Each of these blocks consisted of the following eight trials: two pulse-alone trials, five prepulse trials and one null (no stimulus) trial, in pseudo-random order. The pulse-alone trial consisted of a 120 dB pulse for 30 msec. The five prepulse trials consisted of a 30 msec prepulse at 75, 80, 85, 90 or 95 dB (i.e., 5, 10, 15, 20 or 25 dB above background) followed by a 70 msec delay and then a startle pulse (120 dB, 30 msec). The inter-trial interval was averaged 17 s (range 9–29 s).
For each animal, background startle was defined as the mean startle amplitude from the six null trials; this background value was subtracted from the startle amplitudes for each pulse-alone trial and for each prepulse trial, before any further calculations were performed. ASR was then defined as the mean startle amplitude averaged from the 12 pulse-alone trials. %PPI was defined as (1− (mean startle amplitude on prepulse trial) / ASR)) × 100. For each prepulse intensity, %PPI for the six replicate prepulse trials was averaged and this mean was used as the value for the individual animal.
Behavioral testing was completed and data are included for the two parental strains, for 14 of the 15 A strains and for all 22 of the B strains. The A strain that was excluded was one in which poor breeding limited availability of the animals. A total of 448 mice were tested from February to October, 2000, with 90% of the mice being tested by the end of May. Each week a mix of animals from the various strains was tested, depending on availability of the particular strains. Routinely, only two or three animals for a particular strain were available each week; thus animals from each strain were derived from several litters and were tested at a variety of times within the 9 A.M.–5 P.M. test period. Weiss et al. (1999) have reported that, at least in rats, there is no effect of circadian time on prepulse inhibition of acoustic startle). Animals from the progenitor strains were included in testing from February until the end of May. Data for both ASR and PPI are from an n of 10–15 animals/strain.
Genotyping Procedures
Genomic DNA was prepared and purified from tail biopsies obtained from two animals per strain, and genotyping was performed by PCR-based typing of oligonucleotide primer pairs defining selected microsatellite markers, as previously described in detail (Fortin et al. 2001). Strains were genotyped for 625 microsatellite markers informative for C57BL/6J and A/J, spaced with an average coverage of 2.6 cM throughout the entire genome.
Data Analysis
Mean ASR for the total sample of 448 animals was calculated and PPI data was discarded for any animal whose ASR was below 1.5 SD of the mean. This was done because inhibition of ASR by a prepulse becomes unreliable if the baseline ASR is too low. This criterion caused PPI data to be discarded for two animals from strain A58 and one animal from each of strains A62, A57 and B72, for a total of five animals or 1.1% of the total sample. Data for all 448 animals tested were included in the tabulation of mean ASR values (i.e. no data were discarded for ASR).
To assess reliability of phenotypic data, animals in each strain were sequentially numbered across the testing period and means for the group of odd numbered animals compared with the even numbered. Also, to assess the consistency of phenotypic data across the test period, means for the half of the animals in each strain tested earlier were compared with means for the half tested at a later date. Neither of these split halves analyses revealed any within strain differences in means for either ASR or PPI.
The Kolmogorov-Smirnov 1-sample D statistic was computed for the PPI distributions at each of the five prepulse intensities and for the distribution of ASR in each of the RCLs. None of the distributions was found to deviate significantly from the normal distribution, except for the PPI distribution at 85 dB prepulse intensity in strain A58 (less than 0.5% of the distributions). Between-strain differences in ASR and PPI at different prepulse intensities were assessed using 1-way analyses of variance with mouse strain as grouping factor and ASR and PPI as dependent variables. Post-hoc comparisons of the mean values for the parental strains with the mean value for the corresponding congenic strains were performed using t-tests. Although ANOVA is known to be robust with regard to deviation from the assumption of normality and only a very small fraction of the studied distributions deviated significantly from normality (<0.5%), we conducted a non-parametric analysis of variance (Kruskall-Wallis) to compare the different strains to their parental strain with regard to their PPI at the different prepulse intensities and ASR. The conclusions derived from this non-parametric analysis remained the same as those derived from the ANOVA analyses.
To estimate the broad-sense heritability (the proportion of phenotypic variance accounted for by additive genetic variance, h2) of ASR and PPI at different prepulse intensities, we used the formula h2 = VA/(VA + VE), where VA is the additive genetic variance estimated from half of the variance of the RCS strain means (Falconer 1989; DeFries et al. 1989) and VE is the residual variance estimated from the pooled within strain variance. The minimum number of effective factors (e) was estimated for each group of RCSs with a common genetic background according to the formula e = ln (1−p(r ⩾ 1))/ln (1−0.5k+1) (Mulitze and Baker 1985a,b), where p(r ⩾ 1) is the probability that a RCS contains at least one gene (from the donor parental strain) with a significant effect on the phenotype of that RCS compared with its parental background phenotype, ln is the natural logarithm and k is the number of times the donor parental strain was backcrossed to the recurrent parental strain to generate the RCSs (here, k = 2). p(r ⩾ 1) was estimated as the ratio of strains with mean PPI significantly different from the mean PPI of the recurrent parental strain.
For the QTL analysis, data from all animals, regardless of their genetic background, were entered into a 1-way analysis of variance (ANOVA), where the genotype (A/J homozygous coded 1 and C57BL/6J coded 2) was considered as the grouping factor and ASR or PPI at different prepulse intensities was the dependent variable. The Bonferroni correction was used to control for the risk of increased type 1 error, due to multiple testing. The accepted critical value of p for identification of significant associations was set at 0.00001. This value was obtained by dividing 0.05 by the number of markers (n = 625) and by the number of phenotypes tested (n = 6), i.e. (0.05/(625 × 6) = 0.000013). Because of linkage disequilibrium, the actual number of tests is less than the number of markers evaluated (625), thus this level of significance is stringent. While QTLs so identified must be considered provisional until confirmed by further testing, it should also be noted that p < .00001 is a highly conservative threshold, in comparison to p values considered acceptable, in, for example, QTL analyses using recombinant inbred mouse strains (Crawley et al. 1997; Neumann and Collins 1992; Alexander et al. 1996; Kanes et al. 1996; Phillips et al. 1998). Once a significant association was detected at a given marker by analysis of data from the entire sample of animals, as described above, a secondary ANOVA was conducted separately on animals belonging to either one of the genetic backgrounds, in order to determine the effect of donor genes on a given genetic background. In these secondary analyses, the critical p value was set at .001, because a priori evidence for the linkage of the marker with the phenotype under consideration had already been provided.
Between-genotype differences in PPI at specific markers that were significantly associated with either increased or decreased PPI were analyzed using analyses of variance with genotype as the grouping variable and PPI at each prepulse intensity as the outcome variable.
For each locus showing unique contribution in the modulation of PPI across at least four prepulse intensities, we reanalyzed the data using non-parametric Kruskal-Wallis analysis of variance with genotype as the independent variable and PPI at different prepulse intensities as the dependent variables. The results of this analysis were unchanged compared with the parametric analysis of variance.
The corrected regression R-square derived from a regression analysis, where the independent variables were the linked markers and the dependent variables were the PPIs at prepulse intensity of 85 dB in the animals from different strains, was used to estimate the proportion of variance explained by each locus showing unique contribution in the modulation of PPI across at least four prepulse intensities. In this analysis, we selected PPI at a prepulse intensity of 85 dB because it was the modality that showed the highest heritability.
Results
Phenotypic Data: ASR and PPI
Figure 1 shows mean ASR and mean % PPI at pre-pulse intensities of 75, 80, 85, 90 and 95 dB (hereafter identified as PPI75, PPI80, PPI85, PPI90 and PPI95, respectively), for the two parental strains and 36 RCSs. Although mean ASR did not differ between the two parental strains (F1,22 = 0.35, p = .55), there were significant differences in mean ASR among the 38 strains (F37,410 = 6.28, p < .000001; Figure 1, panel a). All RCSs that deviated significantly from their parental ASR phenotype showed increased ASR, irrespective of whether the strains derived from A/J or C57BL/6J parents. The strain distribution pattern of ASR (with strains arranged according to increasing ASR) was continuous, with none of the strains having a mean ASR orders of magnitude higher than other strains. However, several RCSs showed two to three fold increases in ASR compared with their parental strains (Figure 1, panel a). This pattern of distribution is consistent with the existence of an important genetic control of the ASR phenotype and also suggests that no genes with major effect are involved in controlling ASR. The broad sense heritability of ASR was 20.7%. There were no between-strain differences in mean null responses, among the 38 strains (F37,410 = 1.36, p = .07, data not shown).
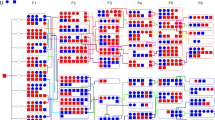
Mean prepulse inhibition (PPI) of acoustic startle responses in recombinant congenic mice separated according to genotype at markers significantly associated with PPI. Markers shown were associated with decreased (panels a, b, c) or increased (panel d) PPI when the A/J gene segregated onto the C57BL/6J genetic background, or with increased PPI when the C57BL/6J gene segregated onto the A/J genetic background (panel e). Comparison is made between the PPI data for the group of recombinant congenic mice carrying A/J versus C57BL/6J alleles at the markers indicated. PPI data for the parental strains, C57BL/6J (C57) and A/J, are also shown for comparison. In panels a, b, and d, categories on the X axis represent groups of mice homozygous for the A/J allele (A) or for the C57BL/6J allele (B). In panel c, where animals were grouped according to their genotypes at two markers, D3Mit189 and D7Mit77, A/A = mice homozygous for the A/J allele at both markers D3Mit189 and D7Mit77, A/B = mice homozygous for the A/J allele at D3Mit189 and for the C57BL/6J allele at D7Mit77, B/B = mice homozygous for the C57BL/6J allele at both markers, and similarly for panel e. Values are means ± SEM. Asterisks indicate values significantly different from B in panels a, b and d, from B/B in panel c and from A/A in panel e, at*p < .05, **p < .01, ***p < .001, ****p < .0001, *****p < .00001.
The A/J and C57BL/6J parental strains showed significant (p < .05) differences in PPI magnitude at all prepulse intensities, except for PPI95 (Figure 1, panels b–g). Significant between-strain differences in mean % PPI were found among the 38 strains, at each prepulse intensity (75 dB: F37,405 = 3.99, p < .000001; 80 dB: F37,405 = 5.46, p < .000001; 85 dB: F37,405 = 6.74, p < .000001; 90 dB: F37,45 = 5.19, p < .000001; 95 dB: F37,405 = 3.84, p < .000001). These between-strain differences were most numerous for PPI80 and PPI85 suggesting a greater degree of genetic control of PPI at these intensities. This was reflected in higher estimates of the broad sense heritabilities at these two intensities (19.6%, 23.4%, respectively) compared with heritabilities for PPI75, PPI90 and PPI95 (15.1%, 18.8% and 15.1% respectively). All RCSs with A/J background, whose PPI was significantly different from that of the parental A/J strain, showed an increase in this trait, irrespective of the intensity of the prepulse used. Conversely, almost all the strains with C57BL/6J background, whose PPI deviated significantly from their parental phenotype, showed a decrease in this trait. These observations indicate that alleles responsible for increased PPI in the C57BL/6J parental strain (compared with the A/J strain) have segregated in some RCSs with A/J background to increase their PPI compared with the parental phenotype and, conversely, alleles responsible for decreased PPI in the A/J inbred strain have segregated in some of the strains with the C57BL/6J background to decrease their PPI. At the higher prepulse intensities (85–95 dB), introduction of A/J genomic segments onto the C57BL/6J background paradoxically increased PPI, relative to the parental C57BL/6J strain, in two RCSs (B75 and B67). As was the case for ASR, the strain distribution patterns for PPI were continuous at each prepulse intensity, suggesting no major gene effect.
In an initial correlational analysis, correlations among ASR and PPI at various prepulses were calculated using mean values for ASR and PPI, from all the RCSs of both parental backgrounds (Table 1, panel a). Correlations between PPI at the various prepulse intensities were all highly significant, and there were no significant correlations between ASR and PPI at any prepulse intensity. In subsequent analyses, correlations were calculated separately for the 14 RCSs with A/J background and for the 22 RCSs with C57BL/6J background, using ASR and PPI data for individual animals to increase the power of the analysis (Table 1, panels b, c). These analyses also revealed highly significant correlations between PPI at the various prepulse intensities, among RCSs of either parental background. For RCSs with the A/J background, ASR was not significantly correlated with PPI, except at PPI75. However, for RCSs with the C57BL/6J background, a significant negative correlation was observed between ASR and PPI at each prepulse intensity.
QTL Analysis
Results of the QTL analysis are shown in Tables 2, 3, 4 . Identified QTLs could be categorized as those significantly associated with PPI but not with ASR (Table 2), with ASR but not PPI (Table 3) or with both PPI and ASR (Table 4). Five QTLs associated with PPI across all five prepulse intensities, but not with ASR, were identified; these included a single QTL on each of chromosomes (chr) 3, 5, and 7 and two QTLs on chr 16 (Table 2). Additional QTLs for PPI only at certain prepulse intensities were also detected; these include QTLs for PPI80 and PPI85 (chr 14, 15), for PPI85 (chr 6, 18) and for PPI90 (chr 1, 2, 17, 19). Several QTLs were associated with ASR alone (chr 1, 3, 8, 9, 12, 14, 15, 18, 19), but not with PPI (Table 3). Finally, two QTLs for both PPI and ASR were found; one of these (chr 2) was associated with PPI at all five prepulse intensities as well as ASR, while the second (chr 11) was associated with PPI at the four highest prepulse intensities and with ASR (Table 4). The associations of these chr 2 and 11 markers with PPI remained highly significant even when effects of ASR were controlled for using analysis of covariance, with the markers as grouping factor, PPI at different prepulse intensities as outcome variable and ASR as covariate. In comparison to the above observed findings, the calculated minimum numbers of effective factors (rounded to the closest integer) on A/J and C57BL/6J genetic backgrounds were estimated to be 7 and 4 for ASR, 1 and 1 for PPI75, 4 and 4 for PPI80, 7 and 3 for PPI85, 2 and 1 for PPI90, and 0 and 1 for PPI95, respectively.
We focused further analyses only on markers significant for PPI across at least four prepulse intensities, with the rationale that these would be the most likely to have important effects on the phenomenon of PPI globally and independent of possible strain variability in auditory function. Table 5 lists the genotypes for all of the mouse strains at the seven QTLs associated with PPI across four or five prepulse intensities. Of these QTLs, four (chr 2, 3, 7, and the proximal QTL on chr 16) appear to be associated with a decrease in PPI in animals with the C57BL/6J genetic background, one (chr 11) with an increase in PPI in animals with the C57BL/6J background and two (chr 5 and distal chr 16) with an increase in PPI in animals with the A/J background.
Inspection of informative RCSs in Table 5 indicates several features of the four loci (chr 2, 3, 7, proximal 16) putatively mediating decreased PPI in animals with the C57BL/6J background. It can be deduced that segregation of A/J alleles at the locus on chr 2 contributed uniquely to the decrease in PPI in strains B82 and B76, because none of the other six loci with significant effect on PPI at most prepulse intensities contained A/J genotypes in these strains. In addition, because strain B83 segregated the A/J genomic locus flanked by markers D2Mit340 and D2Mit493 and did not show significant differences in PPI compared with the C57BL/6J parental strain, it is possible the chr 2 QTL is distal to marker D2Mit493. The proportion of variance in PPI explained by this locus (marker D2Mit113) in animals with the C57BL/6J genetic background was estimated to be 10.7%. Similarly, the locus on chr 3 contributed uniquely to the decreased PPI in strain B73. In addition, strain B70, which segregated the A/J genotype in markers D3Mit106 and D3Mit266, showed no significant change in PPI at any prepulse intensity compared with C57BL/6J, suggesting that the QTL on chr 3 may be in the vicinity of D3Mit189. The proportion of variance in PPI explained by this locus (marker D3Mit189) in animals with the C57BL/6J genetic background was estimated to be 11.5%. Figure 2, panels a and b, shows that animals with the C57BL/6J genetic background, which segregated the A/J allele at either the chr 2 (B82, B76 and B78, n = 33) or chr 3 (B73 and B87, n = 24) locus, have mean PPI significantly lower at all prepulse intensities compared with strains that retained C57BL/6J alleles at these loci (chr 2: F1,260 ratios>12.25, p levels < .0006; chr 3: F1,260 ratios>9.2, p levels < .003) and compared with the C57BL/6J parental strain at PPI75, PPI80, PPI85, PPI90 (chr 2: F1,41 ratios>5.1, p levels < .03; chr 3: F1,32 ratios > 4.2, p levels < .05). Figure 2, panels a and b, also shows that animals with the C57BL/6J genetic background, which segregated A/J alleles at either the chr 2 or chr 3 locus, do not differ from the A/J parental strain with regard to their mean PPI.
Mean acoustic startle responses (ASR) to white noise at 120 dB (a) and prepulse inhibition (PPI) of acoustic startle responses using prepulses of 75 dB (b), 80 dB (c), 85 dB (d), 90 dB (e) and 95 dB (f), in recombinant congenic strains of mice. Values shown are mean ± SEM. In (e), note that a mean value of PPI greater than 100% was calculated for line B67, since startle responses during prepulse trials were slightly lower than the mean null response for some animals. Asterisks indicate strains with values significantly different from that of the parental strain with the same genetic background (i.e. different from A/J for strains labeled A and different from C57BL/6J for strains labeled B) at*p < .05,**p < .01,***p < .001,****p < .0001,****p < .00001.
The proximal locus on chr 16 (D16Mit57 and D16Mit110) did not contribute uniquely to decrease PPI in any of the B strains and it is therefore difficult to assess its specific role. Strain B78 segregated A/J alleles at both the chr 2 and chr 16 loci, while strain B87 segregated A/J alleles at the chr 3, 7 and 16 loci. However, it is of note that strain B77, which segregated A/J genes only at the proximal chr 16 locus (and at one chr 2 marker), did not show significant differences in PPI compared with its background parental strain, suggesting that the proximal chr 16 locus is not a real QTL. It seems likely that the apparent effect of the proximal chr 16 locus (D16Mit57 and D16Mit110) may be largely driven by strain B87, which segregated A/J genotypes at three loci. Consistent with this hypothesis, when strain B87 was excluded from the analysis of variance, the effect of markers D16Mit57 and D16Mit110 was observed only at PPI80 and 85.
The effect of the chr 7 locus on decreasing PPI is also difficult to assess, because the B strain (B87) with decreased PPI carrying A/J alleles at this locus also segregated A/J alleles at the chr 3 and proximal chr 16 loci. However, if a role for the proximal chr 16 QTL is ruled out, as argued above, the effect of the chr 7 QTL remains mainly difficult to disentangle from that of the chr 3 QTL. Animals with the C57BL/6J background, that segregated A/J genes at the chr 3, 7, and proximal 16 loci (strain B87, n = 13), showed significantly decreased PPI compared with strains with the C57BL/6J genetic background that did not segregate A/J genes at the chr 3 and 7 loci (F1,249 ratios>10.5, p levels < .001) and compared with the C57BL/6J parental strain at all prepulse intensities (F1,21 ratios>6.8, p levels < .01) (Figure 2, panel c). Figure 2, panel c, also shows that animals segregating A/J genes at the chr 3 locus but retaining C57BL/6J genes at the chr 7 locus (strain B73, n = 11) have significantly decreased PPI compared with strains with C57BL/6J genetic background (F1,247 ratios>5.7, p levels < .02) and to the C57BL/6J parental strain (F1,19 ratios>7.3, p levels < .02) only at PPI80 and 85. Although none of the strains segregated C57BL/6J genes at the chr 3 locus together with A/J genes at the chr 7 locus, precluding a formal 2-way analysis of variance to test for interaction between these two loci, the data in Figure 2, panel c, suggest that the chr 7 locus may have an effect in reducing PPI in animals with the C57BL/6J background, either additively or in interaction with chr 3. The location of the QTL on chr 7 is likely to be proximal to marker D7Mit247 because strain B69 contained A/J genes at the three markers distal to D7Mit247 and did not show significantly decreased PPI.
The locus on chr 11 is the only one where the A/J genotype was associated with an increased PPI on the C57BL/6J genetic background. This locus contributed uniquely to increase PPI in strains B67 and B75. Figure 2, panel d, indicates that B strains segregating A/J genes at the chr 11 locus (B85, B67, B75, n = 39) had mean PPI significantly higher than that of strains retaining C57BL/6J genes at this locus (F1,260 ratios>9.4, p levels < .003). Although the mean PPI in the B strains segregating A/J genes at chr 11 also tended to be higher than PPI in the C57BL/6J parental strain at all prepulse intensities, these differences did not reach statistical significance. The proportion of variance in PPI explained by this locus (marker D11Mit67) in animals with the C57BL/6J genetic background was estimated to be 6.2%.
Of the two QTLs (chr 5 and distal chr 16) that appeared to be associated with increasing PPI in animals with the A/J genetic background, the locus on chr 5 contributed uniquely to decrease PPI in strain A62. Two recombination events flanking marker D5Mit338 (one in strain A52 and the other in strain A62) restrict the most likely position of the QTL on chr 5 to the vicinity of marker D5Mit338. The proportion of variance in PPI explained by this locus (marker D5Mit338) in animals with the A/J genetic background was estimated to be 6.4%.
Of the informative RCSs with A/J background showing increased PPI relative to the parental A/J strain, all strains that segregated C57BL/6J alleles at the distal chr 16 locus also segregated C57BL/6J alleles at locus 5 (strains A53, A63, A60), making the role of the distal chr 16 locus difficult to assess. Figure 2, panel e, shows mean PPI in animals with the A/J genetic background and C57BL/6J alleles at markers D5Mit338 and D16Mit70. Animals with C57BL/6J alleles at both of these loci (A63, A53, and A60; n = 38) showed significantly increased PPI compared with animals which did not segregate C57BL/6J alleles at either one of these loci (F1,155 ratios>13.3, p levels < .0004) and compared with the A/J parental strain (F1,50 ratios>4.3, p levels < .05) at all prepulse intensities. Also, strains with A/J genetic background, that retained A/J alleles at both the chr 5 and distal chr 16 loci, have mean PPI that is not statistically different from that of the A/J parental strain. Strains with the A/J genetic background which segregated C57BL/6J alleles at the chr 5 locus but retained A/J alleles at the distal chr 16 locus (A52 and A62; n = 24) have significantly increased PPI at all prepulse intensities (except PPI95) compared with strains that retained A/J genes at both of these loci (F1,141 ratios = 4.13, p levels < .05). Although animals with C57BL/6J alleles at both loci showed higher PPI compared with animals with C57BL/6J alleles only at the chr 5 locus, none of these differences were statistically significant. Here again, a formal 2-way analysis of variance could not be performed because no strains with the A/J background segregated C75Bl/6J genes at the distal chr 16 locus while retaining A/J genes at the chr 5 locus. However, these results suggest that the distal chr 16 QTL may have effects in addition to or in interaction with the chr 5 locus (Figure 1, panel e).
DISCUSSION
Overall, our results indicate that ASR and PPI, particularly at prepulse intensities of 80 and 85 dB, are controlled at least in part by genetic factors in A/J and C57BL/6J mice. The observed strain distribution patterns indicate that no major genes are responsible for this genetic control, but, as with most behaviors, these phenotypes are each determined by several genes, termed QTLs. Our provisional mapping indicates that the QTLs involved in PPI are distributed on several chromosomes and some of them may have epistatic interactions. The use of RCS series, in which unlinked genes contributing to a complex trait become separated and fixed in unique haplotype combinations in different strains, can sometimes allow such interacting genes to be studied separately. Indeed, the fact that chr 11 QTL alleles from A/J, the parental strain with low PPI, induced a significant increase in PPI when introduced onto C57BL/6J, the parental strain with high PPI, may indicate that this locus interacts epistatically with other loci (although an additive effect cannot be excluded). The PPI increasing propensity of the chr 11 A/J alleles is either inhibited by interactions with other loci on the A/J background or permitted expression by interactions when introduced on the C57BL/6J background. The presence of such epistatic interactions may indicate that the amount of genetic control estimated on the basis of linear models, such as the one used to estimate heritability in this paper, are, in fact, substantially underestimating the real genetic contribution to this trait.
The highly significant correlations between PPI at various prepulse intensities for the RCSs suggest that there is appreciable overlap in the genetic regulation of PPI across prepulse intensities. This is supported by the observation that several identified QTLs were significant for PPI across all prepulse intensities (Tables 2 and 4). The observation that ASR is correlated with PPI in RCSs with the C57BL/6J (but not the A/J) background is consistent with the identification of some genetic loci associated with both ASR and PPI, and with the observation that such associations are significant almost exclusively in RCSs with the C57BL/6J background (Table 4). ASR and PPI have been reported to exhibit no significant correlation in studies using strain mean data from series of inbred mouse strains and their F1 and F2 progeny (Logue et al. 1997; McCaughran et al. 1997, 1999; Paylor and Crawley 1997). In the present study, the observation that ASR is correlated with PPI in RCSs with C57BL/6J but not A/J background suggests that genes concurrently regulating both phenotypes may be expressed in some strains but not in others. Alternatively, the observed correlation between PPI and ASR in strains with the C57BL/6J genetic background may be due to separate, but linked and cosegregating, genes controlling ASR and PPI at either or both of the chr 2 and 11 loci.
The ASR is the product of a reflex pathway involving the cochlear nucleus, ventrolateral pons, reticular formation and spinal motor neurons (Yeomans and Frankland 1995). Consistent with the minimum number of effective factors estimated to modulate ASR (7 and 4 on A/J and C57BL/6J backgrounds, respectively), we identified seven provisional QTLs increasing ASR on the A/J background and eight on the C57BL/6J. LeRoy et al. (1999) have reported on three provisional QTLs modulating acoustic startle in chromosomal mapping studies with preweanling C57Bl/6By and NZB/B1NJ inbred mice and their F1s and F2s. These QTLs do not overlap with QTLs identified in the present study, possibly due to age differences in experimental animals. In studies with human populations, monozygotic twins have been reported to exhibit a greater degree of similarity in ASR than do dizygotic twins (Carlson et al. 1997), supporting the idea that ASR is under partial genetic control in humans. One gene modulating startle has been identified in humans, the α 1 subunit gene of the inhibitory glycine receptor, mutation in which is responsible for startle disease or hereditary hyperekplexia (Shiang et al. 1993; Andrew and Owen 1997). The chr 11 QTL for ASR in our study (56.00–58.00 cM) is distal to the mouse gene for the glycine receptor α 1 subunit (chr 11, 30.00 cM). However, a QTL for ASR on chr 3 (D3Mit199, 33.7 cM) maps closely to the mouse glycine receptor β subunit gene (chr 3, 36.00 cM), mutations in which have been reported to enhance startle in mouse mutants, suggesting a role in human hyperekplexia (Koch et al. 1996; Becker et al. 2000).
We identified five putative QTLs affecting PPI on the C57BL/6J genetic background. This number is consistent with the estimate of the minimum number of effective factors at PPI80 and PPI85 (4 and 3, respectively) for the C57BL/6J background. In contrast, only two QTLs increasing PPI at most prepulse intensities were identified in strains with the A/J background; this is less than the estimate of effective factors at PPI80 and PPI85 (4 and 7, respectively) for the A/J background. At least two explanations may account for this possible lack of detection of other QTLs on the A/J background. First, the strains with A/J background were less numerous, resulting in reduced power to identify a significant effect of C57BL/6J genes on the A/J background. Second, it is possible that C57BL/6J genes have an effect on PPI restricted to some prepulse intensities. Consistent with the latter hypothesis, loci on chr 6 (D6Mit188 to D6Mit230), chr 15 (D15Mit171 to D15Mit243) and chr 18 (D18Mit186 to D18Mit189) contributed to significantly increase PPI85 on the A/J background. Further studies of these loci affecting PPI at specific prepulse intensities are therefore warranted. In the current study, the estimate of heritability was highest for PPI at prepulse intensities of 80 and 85 dB. This might be because prepulse inhibition is better elicited at these prepulse intensities than at the two extremes of prepulse intensities. Indeed, 75 dB, being near background intensity, elicits less PPI than do the higher prepulse intensities (see Figure 2), while 95 dB may be too high an intensity to be distinguished from the pulse itself. If this is the case, then the measurement of the phenotype will be more robust in the 80–85 dB range than at the extreme prepulses.
QTL mapping using RCSs of mice has not been used previously to map genes involved in behavioral traits and this is the first report identifying provisional QTLs for PPI of the startle response. Although we show here that the RCS system may be very effective for mapping QTLs and revealing potential interactions between them, the fact that all animals are homozygous precludes estimating the relative effect of each QTL on the phenotypic variability and identifying dominance effects. Further experiments, now in progress, with informative test-crosses (F2 derived from backcrossing informative strains to their recurrent parents) will allow for confirmation of currently identified provisional QTLs, give an estimate of their relative effects on phenotypic variability and demonstrate potential dominance. Other experimental approaches, such as the combination of these data and data derived from chromosome substitution mouse strains (Nadeau et al. 2000) may also help to confirm these results and to clarify the role of QTLs that did not contribute uniquely to affect PPI in any of the recombinant congenic strains (e.g. QTL on chr 7).
Examination of genes in the vicinity of the provisional QTLs for PPI reveal several potential candidates of interest, should these QTLs be confirmed. These include genes regulating neurodevelopment such as ephrins A1, A3, and A4, platelet-activating factor acetylhydrolase Ib, integrin α3 and neural cell adhesion molecule 2, genes involved in presynaptic neuronal function such as SNAP-25 and genes involved in regulation of neurotransmission such as G-protein coupled receptor kinase 3 and GABA transporter 1.
An important consideration in assessing acoustic startle responses in mice is the fact that many mouse strains, including C57BL/6J and A/J, suffer from age-related high frequency hearing loss. Using CBA/CaJ as a reference for normal hearing, (Zheng et al. 1999) reported normal auditory brainstem thresholds for C57BL/6J mice until eight months of age, with A/J showing a mild impairment, at higher frequencies, at 8–10 weeks. When compared with their own performance at one month of age, C57BL/6J mice also show a mild high frequency hearing loss by two months of age, which becomes more pronounced by six or seven months (Willott 1986; Li and Borg 1991). However recent studies (McCaughran et al. 1999) indicate that such high frequency hearing deficits do not affect ASR and PPI, if white noise is used as the acoustic stimulus, as in the present study. McCaughran et al. (1999) separated the BXD recombinant inbred series derived from C57BL/6J and DBA/2J parental strains into groups with adult or juvenile (marked loss at < 6 weeks) onset high frequency hearing loss. With white noise as the acoustic stimulus, PPI of acoustic startle was not affected in the juvenile compared with the adult onset group across a range of prepulse intensities, in animals tested at 6–8 weeks of age. Similarly ASR to a 110 dB white noise burst was unaffected by juvenile onset hearing loss. The DBA/2J strain, which demonstrated normal PPI and ASR responses to white noise in the McCaughran et al. (1999) studies, suffers from greater high frequency hearing loss at 8–10 weeks than does the A/J (Zheng et al. 1999) used in the current study. Thus, although some of the QTLs identified in the current study may be related to auditory mechanisms, available evidence strongly suggests that high frequency hearing loss is not an important confound. In this context, (Paylor and Crawley 1997) have reported a significant positive correlation between PPI of responses to acoustic and tactile stimuli in inbred mouse strains, suggesting that at least some genetic substrates regulating these two forms of PPI are similar and independent of hearing ability.
In conclusion, several loci, with possible additive and/or epistatic interactions, are involved in the control of PPI in mice. With rapid progress in the mouse genome project (Nadeau et al. 2001) and knowledge of the neuropharmacology of PPI (Swerdlow et al. 1998), this mapping information in mice may help to identify candidate genes that can be tested for their role in modulating PPI. In addition, human genes homologous to these mouse candidates, especially those human homologs mapping to loci previously linked to schizophrenia, may be tested in schizophrenic patients as candidates for increasing susceptibility to this disease. This approach may help in resolving part of the difficulties encountered in genetic studies of schizophrenia.
References
Alexander RC, Wright R, Freed W . ( 1996 ): Quantitative trait loci contributing to phencyclidine-induced and amphetamine-induced locomotor behavior in inbred mice . Neuro 15 : 484 – 490
Andrew M, Owen MJ . ( 1997 ): Hyperekplexia: abnormal startle response due to glycine receptor mutations . Br J Psychiatry 170 : 106 – 108
Becker L, Hartenstein B, Schenkel J, Kuhse J, Betz H, Weiher H . ( 2000 ): Transient neuromotor phenotype in transgenic spastic mice expressing low levels of glycine receptor beta-subunit: an animal model of startle disease . Eur J Neurosci 12 : 27 – 32
Braff DL, Stone C, Callaway E, Geyer M, Glick I, Bali L . ( 1978 ): Prestimulus effects on human startle reflex in normals and schizophrenics . Psychophysiology 15 : 339 – 343
Braff DL, Swerdlow NR, Geyer MA . ( 1999 ): Symptom correlates of prepulse inhibition deficits in male schizophrenic patients . Am J Psychiatry 156 : 596 – 602
Bullock AE, Slobe BS, Vazquez V, Collins AC . ( 1997 ): Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response . Behav Neurosci 111 : 1353 – 1360
Butler RW, Jenkins MA, Sprock J, Braff DL . ( 1992 ): Wisconsin Card Sorting Test deficits in chronic paranoid schizophrenia. Evidence for a relatively discrete subgroup? Schizophr Res 7 : 169 – 176
Cadenhead KS, Geyer MA, Braff DL . ( 1993 ): Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder . Am J Psychiatry 150 : 1862 – 1867
Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL . ( 2000 ): Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits . Am J Psychiatry 157 : 1660 – 1668
Carlson S, Willott JF . ( 1996 ): The behavioral salience of tones as indicated by prepulse inhibition of the startle response: relationship to hearing loss and central neural plasticity in C57BL/6J mice . Hear Res 99 : 168 – 175
Carlson SR, Katsanis J, Iacono WG, McGue M . ( 1997 ): Emotional modulation of the startle reflex in twins: preliminary findings . Biol Psychol 46 : 235 – 246
Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M . ( 1996 ): Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results . Biol Psychiatry 39 : 33 – 41
Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R . ( 1997 ): Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies . Berl 132 : 107 – 124
DeFries JC, Wilson JR, Erwin VG, Petersen DR . ( 1989 ): LS X SS recombinant inbred strains of mice: initial characterization . Alcohol Clin Exp Res 13 : 196 – 200
Demant P, Hart AA . ( 1986 ): Recombinant congenic strains–a new tool for analyzing genetic traits determined by more than one gene . Immunogenetics 24 : 416 – 422
Ellenbroek BA, Geyer MA, Cools AR . ( 1995 ): The behavior of APO-SUS rats in animal models with construct validity for schizophrenia . J Neurosci 15 : 7604 – 7611
Falconer DS . ( 1989 ): Introduction to quantitative genetics. New York, Ronald Press
Fortin A, Diez E, Rochefort D, Laroche L, Malo D, Rouleau GA, Gros P, Skamene E . ( 2001 ): Recombinant congenic strains derived from A/J and C57BL/6J: a tool for genetic dissection of complex traits . Genomics 74 : 21 – 35
Grillon C, Ameli R, Charney DS, Krystal J, Braff D . ( 1992 ): Startle gating deficits occur across prepulse intensities in schizophrenic patients . Biol Psychiatry 32 : 939 – 943
Kanes S, Dains K, Cipp L, Gatley J, Hitzemann B, Rasmussen E, Sanderson S, Silverman M, Hitzemann R . ( 1996 ): Mapping the genes for haloperidol-induced catalepsy . J Pharmacol Exp Ther 277 : 1016 – 1025
Koch M, Kling C, Becker CM . ( 1996 ): Increased startle responses in mice carrying mutations of glycine receptor subunit genes . Neuroreport 7 : 806 – 808
Kumari V, Soni W, Mathew VM, Sharma T . ( 2000 ): Prepulse inhibition of the startle response in men with schizophrenia: effects of age of onset of illness, symptoms, and medication . Arch Gen Psychiatry 57 : 609 – 614
LeRoy I, Perez-Diaz F, Cherfouh A, Roubertoux PL . ( 1999 ): Preweanling sensorial and motor development in laboratory mice: quantitative trait loci mapping . Dev Psychobiol 34 : 139 – 158
Li HS, Borg E . ( 1991 ): Age-related loss of auditory sensitivity in two mouse genotypes . Acta Otolaryngol 111 : 827 – 834
Light GA, Braff DL . ( 1999 ): Human and animal studies of schizophrenia-related gating deficits . Curr Psychiatry Rep 1 : 31 – 40
Logue SF, Owen EH, Rasmussen DL, Wehner JM . ( 1997 ): Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses . Neuroscience 80 : 1075 – 1086
Marks MJ, Stitzel JA, Collins AC . ( 1989 ): Genetic influences on nicotine responses . Pharmacol Biochem Behav 33 : 667 – 678
McCaughran J, Bell J, Hitzemann R . ( 1999 ): On the relationships of high-frequency hearing loss and cochlear pathology to the acoustic startle response (ASR) and prepulse inhibition of the ASR in the BXD recombinant inbred series . Behav Genet 29 : 21 – 30
McCaughran J, Mahjubi E, Decena E, Hitzemann R . ( 1997 ): Genetics, haloperidol-induced catalepsy and haloperidol-induced changes in acoustic startle and prepulse inhibition . ( Berl 134 : 131 – 139
Mulitze DK, Baker RJ . ( 1985a ): Evaluation of biometrical methods of estimating the number of genes. 1. Effect of sample size . Theor Appl Genet 69 : 553 – 558
Mulitze DK, Baker RJ . ( 1985b ): Evaluation of biometrical methods of estimating the number of genes. 2. Effect of of type I and type II statistical errors . Theor Appl Genet 69 : 559 – 566
Nadeau JH, Balling R, Barsh G, Beier D, Brown SD, Bucan M, Camper S, Carlson G, Copeland N, Eppig J, Fletcher C, Frankel WN, Ganten D, Goldowitz D, Goodnow C, Guenet JL, Hicks G, de Angelis MH, Jackson I, Jacob HJ, Jenkins N, Johnson D, Justice M, Kay S, Kingsley D, Lehrach H, Magnuson T, Meisler M, Poustka A, Rinchik EM, Rossant J, Russell LB, Schimenti J, Shiroishi T, Skarnes WC, Soriano P, Stanford W, Takahashi JS, Wurst W, Zimmer A . ( 2001 ): Sequence interpretation. Functional annotation of mouse genome sequences . Science 291 : 1251 – 1255
Nadeau JH, Singer JB, Matin A, Lander ES . ( 2000 ): Analysing complex genetic traits with chromosome substitution strains . Nat Genet 24 : 221 – 225
Neumann PE, Collins RL . ( 1992 ): Confirmation of the influence of a chromosome 7 locus on susceptibility to audiogenic seizures . Mamm Genome 3 : 250 – 253
Palmer AA, Dulawa SC, Mottiwala AA, Conti LH, Geyer MA, Printz MP . ( 2000 ): Prepulse startle deficit in the Brown Norway rat: a potential genetic model . Behav Neurosci 114 : 374 – 388
Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, Sanfilipo M, Chappell PB, Chakravorty S, Gonzenbach S, Ko GN, Rotrosen JP . ( 2000 ): Impaired prepulse inhibition of acoustic startle in schizophrenia . Biol Psychiatry 47 : 662 – 669
Paylor R, Crawley JN . ( 1997 ): Inbred strain differences in prepulse inhibition of the mouse startle response . Berl 132 : 169 – 180
Perry W, Braff DL . ( 1994 ): Information-processing deficits and thought disorder in schizophrenia . Am J Psychiatry 151 : 363 – 367
Perry W, Geyer MA, Braff DL . ( 1999 ): Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients . Arch Gen Psychiatry 56 : 277 – 281
Phillips TJ, Belknap JK, Buck KJ, Cunningham CL . ( 1998 ): Genes on mouse chromosomes 2 and 9 determine variation in ethanol consumption . Mamm Genome 9 : 936 – 941
Shiang R, Ryan SG, Zhu YZ, Hahn AF, O'Connell P, Wasmuth JJ . ( 1993 ): Mutations in the alpha 1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia . Nat Genet 5 : 351 – 358
Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL . ( 1993 ): A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder . Biol Psychiatry 33 : 298 – 301
Swerdlow NR, Braff DL, Geyer MA . ( 1999 ): Cross-species studies of sensorimotor gating of the startle reflex . Ann N Y Acad Sci 877 : 202 – 216
Swerdlow NR, Geyer MA . ( 1998 ): Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia . Schizophr Bull 24 : 285 – 301
Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A . ( 2001 ): Tactile prepuff inhibition of startle in children with Tourette's syndrome: in search of an “fMRI-friendly” startle paradigm . Biol Psychiatry 50 : 578 – 585
Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR . ( 1995 ): Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington's disease . J Neurol Neurosurg Psychiatry 58 : 192 – 200
Swerdlow NR, Taaid N, Oostwegel JL, Randolph E, Geyer MA . ( 1998 ): Towards a cross-species pharmacology of sensorimotor gating: effects of amantadine, bromocriptine, pergolide and ropinirole on prepulse inhibition of acoustic startle in rats . Behav Pharmacol 9 : 389 – 396
Weike AI, Bauer U, Hamm AO . ( 2000 ): Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients . Biol Psychiatry 47 : 61 – 70
Weiss IC, Feldon J, Domeney AM . ( 1999 ): Circadian time does not modify the prepulse inhibition response or its attenuation by apomorphine . Pharmacol Biochem Behav 64 : 501 – 505
Willott JF . ( 1986 ): Effects of aging, hearing loss, and anatomical location on thresholds of inferior colliculus neurons in C57BL/6 and CBA mice . J Neurophysiol 56 : 391 – 408
Yeomans JS, Frankland PW . ( 1995 ): The acoustic startle reflex: neurons and connections . Brain Res Brain Res Rev 21 : 301 – 314
Zheng QY, Johnson KR, Erway LC . ( 1999 ): Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses . Hear Res 130 : 94 – 107
Acknowledgements
This work was supported by grants to R.J. and P.B. from RGS Genome Inc., the National Alliance for Research on Schizophrenia and Depression (NARSAD) and the Canadian Institutes of Health Research (CIHR). We thank Ying Zhang and Julie Zhu for their expert and careful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joober, R., Zarate, JM., Rouleau, GA. et al. Provisional Mapping of Quantitative Trait Loci Modulating the Acoustic Startle Response and Prepulse Inhibition of Acoustic Startle. Neuropsychopharmacol 27, 765–781 (2002). https://doi.org/10.1016/S0893-133X(02)00333-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(02)00333-0
Keywords
This article is cited by
-
Crybb2 coding for βB2-crystallin affects sensorimotor gating and hippocampal function
Mammalian Genome (2013)
-
Serotonin1A receptor deletion does not interact with maternal separation-induced increases in startle reactivity and prepulse inhibition deficits
Psychopharmacology (2011)
-
Epigenetics and Biomarkers in the Staging of Neuropsychiatric Disorders
Neurotoxicity Research (2010)
-
Selective Breeding of Reduced Sensorimotor Gating in Wistar Rats
Behavior Genetics (2007)
-
A QTL on rat chromosome 7 modulates prepulse inhibition, a neuro-behavioral trait of ADHD, in a Lewis x SHR intercross
Behavioral and Brain Functions (2006)