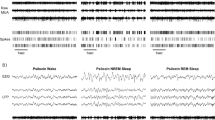
Abstract
The endogenous neurosteroid allopregnanolone has recently been demonstrated to have somnogenic properties that are very similar to those of other agonistic modulators of GABAA receptors, especially of short-acting benzodiazepines. Short-acting benzodiazepines are established to rapidly lose their hypnotic effect upon repeated administration. To investigate the tolerance potential of allopregnanolone, we assessed sleep-wake behavior in rats during subchronic treatment (once daily for five days) with placebo or 15 mg/kg allopregnanolone (n = 8 each). The sleep patterns of the placebo and allopregnanolone group did not differ significantly before and after treatment. Throughout the entire treatment period the allopregnanolone group exhibited shorter non-rapid eye movement sleep (non-REMS) latencies, prolonged REMS latencies, longer non-REMS episodes, more pre-REMS and less low-frequency, but higher spindle activity in the electroencephalogram (EEG) within non-REMS than the placebo group. The lack of tolerance effects suggests that allopregnanolone may be an efficacious modulator of sleep-wake behavior over longer time periods than most drugs targeting the benzodiazepine binding site of the GABAA receptor.
Similar content being viewed by others
Main
Various steroids modulate γ-aminobutyric acid (GABA)A receptor-mediated transmission through an allosteric mechanism that is distinct from that of barbiturates and benzodiazepines. Particularly the ring A-reduced metabolites of progesterone: 3α-hydroxy-5α-pregnan-20-one (allopregnanolone) and 3α-hydroxy-5β-pregnan-20-one (pregnanolone), and of deoxycorticosterone: tetrahydro-DOC (THDOC), are potent naturally occurring agonistic modulators of GABAA receptors (reviewed in Majewska 1992; Rupprecht and Holsboer 1999). Earlier studies demonstrated that these neurosteroids exhibit hypnotic properties. Pregnanolone and THDOC have been shown to rapidly promote non-rapid eye movement sleep (non-REMS) in rats (Edgar et al. 1997; Mendelson et al. 1987). Reportedly, pregnanolone also enhances sleep propensity in humans (Schulz et al. 1996). Likewise, allopregnanolone evokes dose-related sleep changes in rats, including a reduced sleep onset latency, an increase in pre-REMS (a substate of non-REMS, which usually precedes REMS), an attenuation of power in the lower frequencies and an elevation of power in the frequency range of sleep spindles in the electroencephalogram (EEG) within non-REMS (Lancel et al. 1997).
The sleep effects of THDOC, pregnanolone and allopregnanolone closely match those of other agonistic modulators of GABAA receptors, especially of short-acting benzodiazepines. In rats, benzodiazepines are established to shorten the latency to non-REMS, promote particularly pre-REMS, depress slow frequency components and enhance spindling in the electroencephalogram (EEG) within non-REMS (reviewed in Lancel 1999). One notable difference is that benzodiazepines transiently suppress REMS, an effect till now not observed for neurosteroids. A characteristic shared by many agonistic modulators of GABAA receptors is the rapid development of tolerance towards their hypnotic action. For instance, upon acute administration agonistic modulators of GABAA receptors produce a marked sedation-evoked reduction in locomotor activity and exploratory behavior in rodents. Already after three days of chronic dosing, the benzodiazepines lorazepam and triazolam failed to reduce locomotion and exploration in rats (File 1981). Furthermore, the sleep promoting action of triazolam and of a novel non-benzodiazepine hypnotic, zaleplon, was found to vanish within five days of daily drug administration in rats (Crespi 1999; Depoortere et al. 1998). Correspondingly, in humans benzodiazepines lose their somnogenic effect after a few days to weeks of chronic usage (reviewed in Ashton 1994). Moreover, abrupt withdrawal of agonistic modulators of GABAA receptors may produce a transient deterioration of sleep compared to pretreatment levels. This phenomenon—known as rebound insomnia—is particularly reflected by a prolongation of sleep onset latency and a reduction of total sleep time, which may lead to restart hypnotic intake (reviewed in Ashton 1994; Dingemanse 1995; Lader 1992).
To investigate whether allopregnanolone also rapidly produces tolerance towards its hypnotic effects and disrupts sleep upon abrupt drug discontinuation, we assessed sleep-wake behavior in a group of rats before, during and after subchronic administration of allopregnanolone and compared their sleep profiles with those of vehicle-treated animals. Allopregnanolone was administered at the beginning of the dark period, because sleep-wake behavior of the rat during the dark period, which coincides with its daily activity phase, is more sensitive to the sleep promoting effects of drugs.
MATERIALS AND METHODS
Animals
Under halothane anaesthesia, 16 male Wistar rats (Charles River Laboratories, Sulzfeld, Germany), weighing between 290 and 370 g, were implanted with EEG and EMG electrodes as described in detail elsewhere (Lancel et al. 1996). Additionally, an epidural thermistor (Betatherm Ireland Ltd.; type 10K3MCD1) was implanted (position: 1 posterior, 3.5 lateral relative to the bregma) to record brain temperature (Tbr). The animals were housed individually in a ventilated, sound-attenuated Faraday room under a 12-h light/dark schedule (lights on from 06:00 AM) at a regulated ambient temperature of 21–22°C. Food and water were available ad libitum. At least three weeks were allowed to recover from surgery and five days to adapt to the recording conditions.
Experimental Design
The experiment was approved by the local commission for animal welfare. The experiment lasted nine consecutive days, consisting of two baseline days, five treatment days (T1 to T5) and two drug withdrawal days (W1 and W2). On each day, the animals received an intraperitoneal injection 5 min before dark-onset. The rats of the placebo group (n = 8) received vehicle during all days. The rats of the allopregnanolone group (n = 8) were injected with vehicle during the baseline days, 15 mg/kg allopregnanolone (Sigma, Deisenhofen, Germany) on each treatment day and vehicle during both withdrawal days. Allopregnanolone was dissolved in 35% hydroxylpropyl-β-cyclodextrin (1.5 ml/kg, bwt) and was thereafter mixed with corn oil (3 ml/kg, bwt) to prolong its presence. Vehicle consisted of 35% hydroxylpropyl-β-cyclodextrin and corn oil (1.5 and 3 ml/kg, bwt, respectively).
Data Analysis
EEG, EMG and Tbr were continuously recorded during the first 6 h after each injection. The electroencephalogram (EEG) and electromyogram (EMG) signals were amplified and filtered (EEG: high-pass 0.3 Hz and low-pass 29 Hz, 49 dB/octave; EMG: high-pass 16 Hz and low-pass 3000 Hz, 6 dB/octave). EEG, rectified and integrated EMG and Tbr were digitized with a sampling rate of 64 Hz. An off-line program displayed 10-s epochs of EEG and EMG on screen for the manual scoring of the vigilance states wakefulness, non-REMS, pre-REMS and REMS. Pre-REMS, also called the intermediate state, usually precedes REMS and is characterized by high-amplitude spindle-like EEG signals on a background of theta (6–9 Hz) activity (Gandolfo et al. 1994).
The data were scored by K.D. and M.L., who were blind to the treatment. The EEG was subjected to an on-line fast Fourier transform routine. A power spectrum was computed for 2-s windows in 0.5 Hz bins for the frequencies between 0.5 and 4.5 Hz and in 1 Hz bins for the frequencies between 5 and 25 Hz. Power spectra were averaged over 10-s epochs.
For each recording period, the latency to non-REMS and REMS (arbitrarily defined as the 20th 10-s epoch of non-REMS and the 3rd 10-s epoch of REMS), total time spent in each vigilance state and the number and average duration of the non-REMS (pre-REMS included) and REMS episodes were determined and average EEG power densities within wakefulness, non-REMS and REMS computed. Because of large inter-individual differences in absolute EEG power densities, the EEG values were normalized by expressing them as percentage of the EEG power density in the same frequency band and vigilance state during the baseline recordings and were thereafter log transformed.
To reduce data, for each animal mean values of all parameters were computed over the two baseline days, the second and third treatment day (T2 + T3) and the fourth and fifth treatment day (T4 + T5). In order not to lose variance in baseline EEG power densities, the data of the second baseline day were used for the baseline condition. The variables were analyzed by means of a two-factorial analysis of variance (ANOVA) with repeated measures design (Greenhouse Geiser correction), where group was a between-subjects factor (two levels: placebo and allopregnanolone) and condition a within-subjects factor (six levels: baseline, T1, T2 + T3, T4 + T5, W1, and W2). Furthermore, separate ANOVAs were applied to the data of the treatment conditions (T1, T2 + T3 and T4 + T5) for a more detailed analysis of tolerance. Whenever a significant “group by condition” effect was found, tests with contrasts were performed to locate the conditions with significant differences between the groups. As a nominal level of significance α = 0.05 was accepted and adjusted (according to the Bonferroni correction) for all posteriori tests in order to keep the type I error ⩽0.05.
RESULTS
Brain Temperature
Analysis of the 6-h Tbr values revealed a significant effect of condition [F(5,70) = 2.9, p = .05] and of group by condition [F(5,70) = 3.3, p = .03]. Tests with contrasts showed that Tbr was tendentially (p = .07) lower in the allopregnanolone group than in the placebo group on T1, while no differences were found for the other conditions (Figure 1A).
Brain temperature (A) and percentage of time spent in wakefulness (B), non-REMS (C), pre-REMS (D), and REMS (E) during the 6-h baseline (B1 + B2), treatment (T1, T2 + T3, T4 + T5) and drug withdrawal (W1, W2) condition in the placebo and allopregnanolone group. Curves connect mean values ± SEM (n = 8 each). * denotes a significant difference between the groups during a specific condition (p < .05, tests with contrasts).
Vigilance States
ANOVA yielded a significant effect of condition for wakefulness [F(5,70) = 14.8, p < .0001], non-REMS [F(5,70) = 10.5, p < .0001] and REMS [F(5,70) = 6.8, p = .0007]. Irrespective of the group, wakefulness increased across the experimental conditions, which was related to slight reductions in both non-REMS and REMS (Figure 1B, C, and E). For pre-REMS a significant effect of condition [F(5,70) = 4.0, p = .001] and of group by condition [F(5,70) = 4.6, p = .005] emerged.
Tests with contrasts showed that the placebo and allopregnanolone groups displayed comparable amounts of pre-REMS during baseline and both withdrawal days. However, the allopregnanolone group had more pre-REMS during all treatment conditions, significantly on T1 and T4 + T5 and tendentially (p = .09) on T2 + T3 (Figure 1D). To examine whether the promotion of pre-REMS by allopregnanolone varied across the treatment days, a separate ANOVA was applied to the data of the treatment conditions. A significant effect of group [F(1,14) = 7.0, p = .02] but not of condition or of group by condition was found, which indicates that the allopregnanolone-evoked increase in pre-REMS did not diminish across the treatment period.
Sleep Architecture
Analysis of the latency to non-REMS yielded a significant effect of group [F(1,14) = 7.1, p = .02]. The allopregnanolone group generally exhibited shorter non-REMS latencies than the placebo group, most prominently during the treatment conditions (Figure 2A). For the number of non-REMS episodes a significant group effect was found [F(1,14) = 6.3, p = .03]. Overall, the allopregnanolone group had a smaller number of non-REMS episodes, particularly during the treatment conditions (Figure 2B). Additionally, a significant condition effect emerged [F(5,70) = 2.7, p = .05], reflecting a moderate, group-independent reduction in the number of non-REMS episodes across the experimental conditions. For the duration of the non-REMS episodes a significant group [F(1,14) = 6.2, p = .03], condition [F(5,70) = 7.4, p < .0001] and a group by condition [F(5,70) = 4.4, p = .006] effect was observed. During baseline non-REMS episode duration did not differ between the groups. On T1 the allopregnanolone group tended (p = .1) to have longer episodes and this effect was significant on T2 + T3 and T4 + T5. During the withdrawal days the allopregnanolone group reached placebo levels again (Figure 2C). Analysis of the latency to REMS revealed a significant effect of group [F(1,14) = 9.1, p = .009], condition [F(5,70) = 2.7, p = .05], and of group by condition [F(5,70) = 3.9, p = .01]. Compared to the placebo group, the allopregnanolone group had a significantly longer REMS latency on all treatment conditions, while no difference was found for baseline or withdrawal days (Figure 2D). A significant condition effect [F(5,70) = 5.5, p = .0003] emerged for the number of REMS episodes, which was due to a moderate decrease over the experimental days that occurred in both groups (Figure 2E). Analysis of the duration of the REMS episodes did not yield significant effects (Figure 2F). Separate ANOVA of the treatment conditions revealed a significant effect of group for the latency to non-REMS [F(1,14) = 11.5, p = .005], the number and duration of the non-REMS episodes [F(1,14) = 7.6, p = .02 and F = 9.6, p = .008, respectively] and the latency to REMS [F(1,14) = 13.0, p = .003] but not of condition or group by condition, suggesting that the influence of allopregnanolone on these variables did not decline across the treatment conditions.
Latency to non-REMS (A), number (B), duration (C) of non-REMS episodes; latency to REMS (D), number (E), and duration (F) of REMS episodes during the 6-h baseline (B1 + B2), treatment (T1, T2 + T3, T4 + T5) and drug withdrawal (W1, W2) condition in the placebo and allopregnanolone group. Curves connect mean values ± SEM (n = 8 each). *---* denotes a significant main effect of group (p < .05, ANOVA) and * a significant difference between the groups during a specific condition (p < .05, tests with contrasts)
EEG Power Densities within Non-REMS
Analysis of the 6-h normalized and log transformed EEG power densities within non-REMS revealed a significant (p < .05) effect of group for the frequencies from 0.5 up to 3.5 Hz, reflecting overall differences between the groups. As can be seen in Figure 3, low-frequency activity in the allopregnanolone group was lower than in the placebo group primarily during the treatment conditions. ANOVA yielded a significant effect of condition for the frequencies between 0.5 and 4.5 Hz, which was due to a slight, group-independent decrease across the experimental conditions. Further, a significant condition and group by condition effect was found for all frequencies ⩾8 Hz. Tests with contrasts showed that high-frequency activity in the allopregnanolone group exceeded that in the placebo group during T1, T2 + T3 and T4 + T5. On the two drug withdrawal days the allopregnanolone and placebo group exhibited comparable power densities again. Pairwise comparisons per 2-h interval showed that all effects of allopregnanolone on the EEG within non-REMS were most pronounced during the first 2-h interval after injection and gradually diminished thereafter (data not shown).
EEG power densities within non-REMS during the 6-h baseline (B2), treatment (T1, T2 +T3, T4 + T5), and drug withdrawal (W1, W2) conditions in the placebo and allopregnanolone group. Curves connect mean values ± SEM (n = 8 each). For plotting purposes, the data are expressed as a percentage of the average power densitiy in the same frequency band and vigilance state during the two baseline days. Lines at the bottom of the graphs indicate frequency bands in which EEG power density differed significantly between the groups (p < .05, tests with contrasts run on normalized and log transformed values).
ANOVA performed on the data of the treatment period revealed a significant group effect for all frequency bands below 4.5 Hz and above 8 Hz, whereas no significant effect of group by treatment condition emerged.
EEG Power Densities within REMS
For the EEG power densities within REMS ANOVA yielded a significant effect of condition for the frequencies between 1.5 and 7 Hz, which was caused by a general attenuation of power in the respective frequency bands in the course of the experiment. For the 6 Hz band, ANOVA found a significant group by condition effect. Compared to the placebo group, the allopregnanolone group displayed significantly more power in this low-theta band on T1 and T2 + T3 (Figure 4). Furthermore, significant group by condition effects emerged for all frequencies ⩾10 Hz. Tests with contrasts showed that high-frequency activity was higher in the allopregnanolone group than in the placebo group during all treatment conditions, but lower during the withdrawal days. Separate analysis of the treatment conditions yielded a significant effect of group for 6 Hz and all frequencies above 9 Hz as well as a tendential (p < .1) or significant interaction effect between the factors group and treatment condition for some of the higher frequency bands, which reflect a decrease in allopregnanolone-evoked enhancements from T2 + T3 to T4 + T5.
EEG power densities within REMS during the 6-h baseline (B2), treatment (T1, T2 + T3, T4 + T5) and drug withdrawal (W1, W2) conditions in the placebo and allopregnanolone group. Curves connect mean values ± SEM (n = 8 each). See legend to Figure 3.
EEG Power Densities within Wakefulness
Analysis of the waking EEG yielded a significant condition effect for the frequencies between 0.5 and 4 Hz. Similar to non-REMS and REMS, low-frequency activity within wakefulness decreased in the course of the experiment in both groups of rats. ANOVA also found a significant condition and group by condition effect for the frequency bands ⩾12 Hz. On T1, high-frequency activity was markedly higher in the allopregnanolone than in the placebo group (Figure 5). This effect of allopregnanolone subsided across the treatment conditions. During the withdrawal days EEG power densities were practically identical in the allopregnanolone and placebo group.
EEG power densities within wakefulness during the 6-h baseline (B2), treatment (T1, T2 + T3, T4 + T5) and drug withdrawal (W1, W2) conditions in the placebo and allopregnanolone group. Curves connect mean values ± SEM (n = 8 each). See legend to Figure 3.
DISCUSSION
The results of the present study confirm the previously reported sleep effects of allopregnanolone in the rat and demonstrate that these effects are sustained during subchronic dosing (daily administration during five days). The latter observation indicates that this naturally occurring neuroactive steroid, in contrast to many synthetic agonistic modulators of GABAA receptors, may not rapidly develop tolerance towards its soporific activity in the rat.
The sleep profile of the placebo group is in good agreement with the literature. Rats are nocturnal animals that sleep little, highly fragmented and - as indexed by low levels of EEG slow wave activity - shallow during darkness (Borbély and Neuhaus 1979; Lancel and Kerkhof 1989). Although these features remained present, sleep-wake behavior slightly changed across the experimental days, in that the amount of wakefulness increased, which was associated with a reduction in the number of non-REMS and REMS episodes, and low-frequency activity in the EEG during waking, non-REMS and REMS decreased. The latter may be due to the build-up of connective tissue around the EEG electrodes, which attenuates the amplitude of the EEG signals as a function of time. An earlier study also revealed that the total amount of REMS, the number of REMS episodes and low-frequency EEG activity gradually decline during long-term recordings in rats (Lancel and Langebartels 2000).
In accordance with previous observations in rats (Lancel et al. 1997), 15 mg of allopregnanolone clearly promoted sleep in that it reduced the latency to non-REMS, decreased the number, but markedly lengthened the duration of the non-REMS episodes and increased the amount of pre-REMS. These findings indicate that allopregnanolone increases the ability to fall and to stay asleep. Moreover, quantitative EEG analysis confirmed that allopregnanolone attenuates slow frequency components, while enhancing EEG power in the frequency range of sleep spindles as well as in the higher frequency bands within non-REMS. The present study demonstrates that all effects of allopregnanolone on sleep architecture and on sleep-EEG persisted during 5 days of daily treatment. The finding that allopregnanolone decreased Tbr only on T1 shows that the allopregnanolone-induced promotion of sleep is not secondary to its influence on Tbr.
Previous research found no effect of THDOC, pregnanolone and allopregnanolone on the temporal evolution of REMS (Edgar et al. 1997; Lancel et al. 1997; Mendelson et al. 1987), which suggested that neuroactive steroids may not interfere with neuronal processes governing the initiation or maintenance of this state. However, the present study shows that allopregnanolone, administered at dark-onset, powerfully and persistently delayed the latency to REMS. The absence of measurable effects on REMS in the afore mentioned studies may be due to the fact that the substances were administered either at the beginning of the light period or in the middle of the dark period, i.e., at times of the day when REMS propensity in the rat is low. In accordance with an earlier study (Lancel et al. 1997), allopregnanolone prominently changed EEG activity within REMS, consisting of an enhancement of low-theta and high-frequency activity. Because the latter was also observed in the EEG within non-REMS and wakefulness, this reflects a largely state-independent alteration in neuronal activity. In contrast to the allopregnanolone-induced changes in sleep architecture, the overall augmentation of high-frequency activity seems to diminish across the treatment days.
The sleep profile induced by alloprenanolone is very reminiscent of that evoked by other agonistic modulators of GABAA receptors, especially short-acting benzodiazepines. In rats, benzodiazepines reduce sleep latency, promote non-REMS (in particular its substate pre-REMS) and transiently suppress REMS (reviewed in Lancel 1999). Furthermore, such compounds depress slow frequency components and augment spindling in the EEG within non-REMS, while enhancing high-frequency activity in all vigilance states (Hashimoto et al. 1992; Lancel et al. 1996; Mandema et al. 1991). However, various agonistic modulators of GABAA receptors, including short-acting benzodiazepines, have been shown to rapidly lose their soporific action upon repeated dosing. For instance, the sedative effect of temazepam, lorazepam, triazolam, and zaleplon steadily vanishes and is completely abolished within three to seven days of subchronic treatment in mice or rats (Crespi 1999; Depoortere et al. 1998; File 1981; Marshall et al. 1997).
With the possible exception of the selective benzodiazepine type I receptor agonists zaleplon and zolpidem (reviewed in Landolt and Gillin 2000), short-acting agonistic modulators of GABAA receptors rapidly induce tolerance in humans too (reviewed in Ashton 1994). Thus, the present finding that the sleep promoting effect of allopregnanolone is sustained and even barely diminishes over five days of daily drug treatment in rats is surprising. Nevertheless, recent data showed that chronic treatment of rats with a synthetic analog of pregnanolone does not induce tolerance to the anticonvulsant action of the steroid itself, while it reduces the anticonvulsant potency of the benzodiazepine diazepam (Reddy and Rogawski 2000). It has to be considered that agonistic modulators of GABAA receptors, such as benzodiazepines, act through a saturable allosteric binding site at the receptor protein, while such a binding site has not been identified for neurosteroids so far. Instead, recent evidence has emerged that steroids may modulate ligand-gated ion channels via an interaction at the receptor-membrane interface (reviewed in Rupprecht and Holsboer 1999). Thus, different modes of action at the molecular level may help to explain the difference in tolerance potential between the neurosteroid allopregnanolone and benzodiazepines.
It is well established that abrupt withdrawal of GABAergic hypnotics in humans often produces a worsening of sleep compared to pretreatment levels that lasts a couple of nights. This so called rebound insomnia, which is presumably related to physical dependence, is especially associated with difficulties in falling and/or staying asleep (reviewed in Ashton 1994; Dingemanse 1995; Lader 1992). To assess the effects of abrupt allopregnanolone withdrawal, we also investigated sleep-wake behavior in the rat during the first two days after drug discontinuation. Although the sleep patterns of the placebo and allopregnanolone group did not differ significantly, the allopregnanolone group tended to display more wakefulness, most prominently during the second withdrawal day. This tendency implies that allopregnanolone withdrawal may transiently disrupt sleep. Such a withdrawal effect would be in line with previous studies in rats showing that allopregnanolone withdrawal - produced by withdrawal from chronic exposure to progesterone- increases neuronal excitability, which is due to a decrease in GABAergic inhibition (Smith et al. 1998) and reflected by increased anxiety (Gallo and Smith 1993).
In conclusion, our data suggest that in rats allopregnanolone, administered in a dose that reliably promotes sleep, may have a lower tolerance potential than many other agonistic modulators of GABAA receptors, including benzodiazepine hypnotics. If our present findings are transferable to humans, neurosteroids, such as allopregnanolone, may offer new therapeutic options for the treatment of sleep onset and sleep maintenance insomnia.
References
Ashton H . (1994): Guidelines for the rational use of benzodiazepines. When and what to use. Drugs 48: 25–40
Borbély AA, Neuhaus HU . (1979): Sleep-deprivation: effects on sleep and EEG in the rat. J Comp Physiol 133: 71–87
Crespi F . (1999): Cholecystokinin-B (CCK-B) receptor antagonists improve “aged” sleep: A new class of sleep modulators? Methods Find Exp Clin Pharmacol 21: 31–38
Depoortere H, Decobert M, Perrault G, Françon D, Sanger DJ . (1998): Neuropharmacological profile of zaleplon, a novel BZ1(ω1) hypnotic, in rodents. J Sleep Res 7(Suppl 2):64
Dingemanse J . (1995): Pharmacotherapy of insomnia: Practice and prospects. Pharm World Sci 17: 67–75
Edgar DM, Seidel WF, Gee KW, Lan NC, Field G, Xia H, Hawkinson JE, Wieland S, Carter RB, Wood PL . (1997): CCD-3693: An orally bioavailable analog of the endogenous neuroactive steroid, pregnanolone, demonstrates potent sedative hypnotic actions in the rat. J Pharmacol Exp Ther 282: 420–429
File SE . (1981): Rapid development of tolerance to the sedative effects of lorazepam and triazolam in rats. Psychopharmacology 73: 240–245
Gallo AM, Smith SS . (1993): Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: A possible rat model of PMS anxiety. Pharmacol Biochem Behav 46: 897–904
Gandolfo G, Scherschlicht R, Gottesman C . (1994): Benzodiazepines promote the intermediate stage at the expense of paradoxical sleep in the rat. Pharmacol Biochem Behav 49: 921–927
Hashimoto T, Hamada C, Wada T, Fukuda N . (1992): Comparative study on the behavioral and EEG changes induced by diazepam, buspirone and a novel anxioselective anxiolytic, DN-2327, in the cat. Neuropsychobiology 26: 89–99
Lader M . (1992): Rebound insomnia and newer hypnotics. Psychopharmacology 108: 248–255
Lancel M . (1999): Role of GABAA receptors in the regulation of sleep: Initial sleep responses to peripherally administered modulators and agonists. Sleep 22: 33–42
Lancel M, Crönlein TAM, Faulhaber J . (1996): Role of GABAA receptors in sleep regulation: Differential effects of muscimol and midazolam on sleep in rats. Neuropsychopharmacology 15: 63–74
Lancel M, Faulhaber J, Schiffelholz T, Romeo E, Di Michele F, Holsboer F, Rupprecht R . (1997): Allopregnanolone affects sleep in a benzodiazepine-like fashion. J Pharmacol Exp Ther 282: 1213–1218
Lancel M, Kerkhof GA . (1989): Effects of repeated sleep deprivation in the dark- or light-period on sleep in rats. Physiol Behav 45: 289–297
Lancel M, Langebartels A . (2000): γ-Aminobutyric acidA (GABAA) agonist 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridin-3-ol persistently increases sleep maintenance and intensity during chronic administration to rats. J Pharmacol Exp Ther 293: 1084–1090
Landolt HP, Gillin JC . (2000): GABAA1a receptors: Involvement in sleep regulation and potential of selective agonists in the treatment of insomnia. CNS Drugs 13: 185–199
Majewska MD . (1992): Neurosteroids: Endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol 38: 379–395
Mandema JW, Sansom LN, Dios-Vièitez MC, Hollander-Jansen M, Danhof M . (1991): Pharmacokinetic-pharmacodynamic modelling of the EEG effects of benzodiazepines. Correlations with receptor binding and anticonvulsant activity. J Pharmacol Exp Ther 257: 472–478
Marshall FH, Stratton SC, Mullings J, Ford E, Worton SP, Oakley NR, Hagan RM . (1997): Development of tolerance in mice to the sedative effects of the neuroactive steroid minaxolone following chronic exposure. Pharmacol Biochem Behav 58: 1–8
Mendelson WB, Martin JV, Perlis M, Majewska MD, Paul SM . (1987): Sleep induction by an adrenal steroid in the rat. Psychopharmacology 93: 226–229
Reddy DS, Rogawski MA . (2000): Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther 295: 1241–1248
Rupprecht R, Holsboer F . (1999): Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci 22: 410–416
Schulz H, Jobert M, Gee KW, Ashbrook DW . (1996): Soporific effect of the neurosteroid pregnanolone in relation to the substance's plasma level: A pilot study. Neuropsychobiology 34: 106–112
Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X . (1998): GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392: 926–930
Acknowledgements
We are grateful to Arnold Höhne for his excellent technical assistance. This study was supported by grants of the Deutsche Forschungsgemeinschaft (M.L.) and the Gerhard-Heβ Programm of the Deutsche Forschungsgemeinschaft (R.R.).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Damianisch, K., Rupprecht, R. & Lancel, M. The Influence of Subchronic Administration of the Neurosteroid Allopregnanolone on Sleep in the Rat. Neuropsychopharmacol 25, 576–584 (2001). https://doi.org/10.1016/S0893-133X(01)00242-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(01)00242-1