Abstract
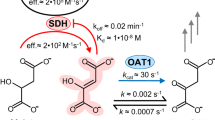
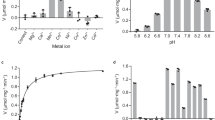
IT is generally believed that aldehyde mutase (the enzyme which catalyses the dismutation of aldehydes in accordance with reaction (1)) is identical with aldehyde oxidase, which oxidizes aldehydes in accordance with reaction (2): where A may be O2, methylene blue or some other hydrogen acceptor. Wieland1 suggests that the oxidase normally uses a hydrogen acceptor to produce an oxidation of the aldehyde, but when no other acceptor is present it uses a second molecule of aldehyde as acceptor, reducing it to alcohol and so producing a dismutation of aldehyde (reaction 1).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wieland, H., Ber. deutsch. chem. Ges., 47, 2085 (1914).
Dixon, M., and Thurlow, S., Biochem. J., 18, 971 (1924).
Dixon, M., and Kodama, K., Biochem. J., 20, 1104 (1926).
Reichel, L., and Köhle, H., Z. physiol. Chem., 236, 145 (1935).
Euler, H. v., and Brunius, E., Z. physiol. Chem., 175, 52 (1928).
Lohmann, K., Biochem. Z., 254, 332 (1932).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DIXON, M., LUTWAK-MANN, C. Aldehyde Mutase. Nature 139, 548–549 (1937). https://doi.org/10.1038/139548b0
Issue Date:
DOI: https://doi.org/10.1038/139548b0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.