Abstract
Serotonergic receptors of the 5-HT1A subtype have been suggested to play a pivotal role in the mechanism of action of antidepressant drugs, including specific serotonin reuptake inhibitors (SSRIs). We examined the effect of clinical doses of the SSRI, fluoxetine, on 5-HT1A receptor function in 15 normal volunteers. Hypothermic and hormone responses to the 5-HT1A receptor agonist, ipsapirone (0.3 mg per kg, per os) were examined after two weeks of placebo and again, after the subjects had been receiving fluoxetine for four weeks. On fluoxetine, the hypothermic response to ipsapirone was significantly blunted, as were ACTH, cortisol and growth hormone release. Ipsapirone plasma levels were significantly increased by fluoxetine but a pharmacokinetic effect could not have accounted for the observed blunting of 5-HT1A receptor mediated effects. These findings confirm and extend previous observations in rodents and humans and indicate that both post-synaptic 5-HT1A receptors in the hypothalamus, which mediate hormone responses to 5-HT1A agonists, and pre-synaptic 5-HT1A receptors which (putatively) mediate the hypothermic response, are rendered subsensitive by chronic SSRI administration. Since fluoxetine did not have significant effects on mood and other psychological variables in these subjects, alterations in 5-HT1A receptor function induced by SSRIs may have psychotropic relevance only in the context of existing perturbations of serotonergic function which underlie the psychopathological states in which these drugs are therapeutically effective.
Similar content being viewed by others
Main
There is considerable interest in the role of serotonergic (5-HT) receptors of the 5-HT1A subtype in the therapeutic action of antidepressant drugs. 5-HT1A receptors are located presynaptically on serotonergic cell bodies in the raphe nuclei where they serve as autoreceptors which inhibit cell firing and reduce 5-HT release by a negative feedback mechanism, and post-synaptically in the hippocampus, cortex and other brain areas, including the hypothalamus. Based on electrophysiological studies in rats, De Montigny and colleagues (Blier and de Montigny 1994; Mongeau et al. 1997) proposed that each major class of antidepressants increases 5-HT neurotransmission by a distinct mechanism involving either pre- or post-synaptic 5-HT1A receptors. The selective serotonin re-uptake inhibitors (SSRIs), which raise 5-HT synaptic levels when given acutely, were proposed to increase 5-HT transmission on repeated administration by inducing desensitization of somatodendritic 5-HT1A autoreceptors in the raphe nuclei and nerve terminal 5-HT1B autoreceptors, which also mediate feedback inhibition of 5-HT release. These effects would lead to a slow increase in 5-HT levels which corresponds to the delayed clinical effect of the drugs.
Evidence for subsensitivity of somatodendritic 5-HT1A autoreceptors after chronic administration of SSRIs has been provided by microdialysis experiments in which 5-HT levels are measured in vivo in response to a challenge dose of the 5-HT1A receptor agonist, 8-hydroxy-2 (di-n-propylamino) tetralin (8-OH-DPAT) (Rutter et al. 1994, Kreiss and Lucki 1995; Invernizzi et al. 1994). However, other studies using SSRIs (Hjorth and Auerbach 1994; Bosker et al. 1995a,b; Invernizzi et al. 1995, 1996) or clomipramine (Gur et al. 1999) failed to show any change in 5-HT1A autoreceptor subsensitivity by this method. An increase in basal 5-HT levels after chronic SSRI administration, taken to be due to pre-synaptic 5-HT1A and 5-HT1B receptor desensitization, has been observed in cortex (Bel and Artigas 1993), hippocampus (Auerbach and Hjorth 1995) diencephalon (Rutter et al. 1994) and prefrontal cortex (Tanda et al. 1996) and, after chronic administration of clomipramine, in frontal cortex but not hippocampus (Gur et al. 1999). A number of other studies have failed, however, to show increases in basal 5-HT levels in terminal areas after chronic administration of 5-HT uptake blocking drugs (unless the measurements were performed less than 24 hr after the last administration of the drug, in which case the effect is very likely due to the persistence of residual drug) (e.g., Hjorth and Auerbach 1994; Bosker et al. 1995a, b; Invernizzi et al. 1995, 1996).
There is relatively little evidence from binding studies for changes in the number of 5-HT1A receptors after chronic SSRI administration. Welner et al. (1989) observed a reduction in the dorsal raphe and decreased binding in midbrain was observed by Li et al. (1994) after chronic fluoxetine. Changes have been reported by some authors in the hippocampus but are inconsistent, showing increased density (Klimek et al. 1994) or decreased density and increased affinity (Maj et al. 1996). However, Hensler et al. (1991) failed to find binding changes in any brain area after chronic administration of the SSRIs, citalopram and sertraline, and a similar result was obtained by Li et al. (1997) with paroxetine.
A post-synaptic 5-HT1A receptor-mediated response which has been studied after long-term antidepressant treatment in animals, is inhibition of forskolin-stimulated adenylate cyclase activity in hippocampal membranes. Work from our laboratory (Newman and Lerer 1988; Newman et al. 1990, 1992) showed subsensitivity of this response after a variety of antidepressant treatments including ECS, the tricyclic drug, desipramine, and fluoxetine.
The effects of 5-HT1A receptor agonists on temperature regulation and hormone release provide a useful avenue for evaluating the functional sensitivity of 5-HT1A receptors in animals and man. 8-OH-DPAT induces dose dependent hypothermia in rodents which is antagonized by stereoselective 5-HT1A receptor blockade (Goodwin et al. 1985a, 1987a; Hillegaart 1991). Although a presynaptic mechanism has been suggested (Goodwin et al. 1985a, 1987a; Hillegaart 1991), the majority of the evidence indicates that the hypothermic effect of 8-OH-DPAT is mediated via postsynaptic 5-HT1A receptors in the hypothalamus (Hjorth 1985; Hutson et al. 1987; O'Connell et al. 1992; Millan et al. 1993). 5-HT1A receptor stimulation also evokes ACTH and corticosterone release in rodents, via an effect on corticotrophin releasing factor in the paraventricular nucleus of the hypothalamus (Koenig et al. 1987; Gilbert et al. 1988; Haleem et al. 1989; Bagdy and Makara 1994) and possibly through an action at the level of the pituitary as well (Chaouloff 1993).
Ipsapirone, a centrally acting pyrimidinylpiperazine derivative, (Traber and Glaser 1987; Cutler et al. 1993), has a radioligand binding profile very similar to that of 8-OH-DPAT (Peroutka 1988). Orally administered ipsapirone (0.3 mg/kg) induces a hypothermic response in normal human subjects (Lesch et al. 1990a; Gelfin et al. 1995). The hypothermia is dose-related, attenuated by the non-selective 5-HT receptor antagonist, metergoline, completely antagonized by the non-selective β adrenoceptor but stereoselective 5-HT1A/5-HT1B receptor antagonist, pindolol, and not affected by the selective β1 adrenoceptor antagonist, betaxolol (Lesch et al. 1990a). Ipsapirone-induced hypothermia is negatively related to age with no difference between males and females (Gelfin et al. 1995). Ipsapirone also induces release of ACTH, cortisol (Lesch et al. 1989, 1990b; Gelfin et al. 1995), and growth hormone (Lesch et al. 1989) in normal human subjects. The effects of age and gender on ipsapirone-induced ACTH and cortisol release are complex; whereas both responses diminished with age in male subjects, they increased with age in females (Gelfin et al. 1995). Hypothalamic-pituitary-adrenal (HPA) activation by ipsapirone is dose related and pharmacologically attributable to 5-HT1A receptor activation, as indicated by the attenuating effect of metergoline, complete antagonism of the response by pindolol and absence of an effect of betaxolol (Lesch et al. 1990b).
The effect of SSRI administration on temperature, behavioral and hormone responses to 5-HT1A receptor agonists, including ipsapirone, has been examined in a number of rodent studies. The hypothermic response to 8-OH-DPAT was found to be blunted in mice and rats (Goodwin et al. 1985b, 1987b; Hensler et al. 1991; Maj and Moryl 1992).
Examining hormone responses to 5-HT1A agonists in rats, Li et al. (1993, 1996, 1997) showed that ACTH, corticosterone and oxytocin responses to challenge with 8-OH-DPAT were reduced after chronic administration of the SSRIs, fluoxetine and paroxetine, and the response to ipsapirone was reduced after fluoxetine (Li et al. 1994). However, unchanged hormone responses to 8-OH-DPAT after chronic administration of sertraline (O'Donnell and Grealy 1992) and citalopram (Przegalinski et al. 1989) have been reported. Li et al. (1996, 1997) complemented their neuroendocrine challenge studies with measurements of Gi and Go proteins and found a reduction in Gi3 levels in the hypothalamus which paralleled the time course of the fluoxetine and paroxetine effect on hormone responses. In the absence of a change in hypothalamic 5-HT1A receptor binding, these post receptor changes were suggested as mediating desensitization of the 5-HT1A receptor mediated hormone responses.
Studies in humans have yielded results which generally parallel the effect of SSRIs on 5-HT1A receptor mediated temperature and hormone responses in rats. Lesch et al. (1991) found that chronic fluoxetine administration to patients with obsessive compulsive disorder (OCD) resulted in reduced hypothermic, ACTH and cortisol responses to ipsapirone challenge. In normal volunteers, the growth hormone response to buspirone was significantly blunted after sub-chronic fluvoxamine administration (Anderson et al. 1996). Blunting of the hypothermic, cortisol, and growth hormone responses to gepirone was observed by Sargent et al. (1997) in normal volunteers who were receiving paroxetine and of the ACTH and cortisol responses to ipsapirone in normal volunteers on sub-chronic fluoxetine by Berlin et al. (1998) (although it should be noted that in the latter study hormone responses before and during fluoxetine treatment were not directly compared).
Studies using other neuroendocrine challenges in humans have yielded results which at first sight appear opposite to those obtained with 5-HT1A receptor agonists. The cortisol response to the 5-HT precursor 5-hydroxytryptophan (5-HTP) was enhanced in patients with major depression compared to control subjects (Meltzer et al. 1984) and further increased after subchronic treatment with fluoxetine but not with tricyclic antidepressants (Meltzer et al. 1997). This discrepancy is probably due to the fact that the 5-HTP induced rise in cortisol is not mediated by 5-HT1A receptors, since in norman human volunteers pindolol inhibited the prolactin but not the cortisol response to this agent (Meltzer and Maes 1995).
We examined the effect of fluoxetine administration for four weeks to normal volunteers, on temperature, ACTH, cortisol and growth hormone responses to ipsapirone challenge. Based on previous findings, we hypothesized that both the temperature and hormone responses would be blunted by fluoxetine. These findings would reflect subsensitivity of post-synaptic 5-HT1A receptors in the hypothalamus (subserving the hormone responses) and of somatodendritic 5-HT1A autoreceptors (if hypothalamic responses to 5-HT1A agonists are pre-synaptically medicated) following chronic SSRI administration.
METHOD
Subjects
Fifteen volunteers were recruited from the hospital staff. Their mean (±standard deviation, SD) age was 38.5 ± 12.9 years (range 22–63) and weight 72.3 ± 10.2 kg; 9 were male and 6 were female. All gave written informed consent for the study which had been approved by the Helsinki Committee (Internal Review Board) of the Hadassah–Hebrew University Medical Center. They were interviewed with the Schedule for Affective Disorders and Schizophrenia–Lifetime Version (Spitzer and Endicott 1978) and did not meet criteria for any DSM-IIIR Axis I or II diagnosis (American Psychiatric Association 1987). They had no history of psychiatric illness in first degree relatives, were in good physical health, were not taking medication of any type and did not use any substances (besides nicotine in 7 cases).
Fluoxetine Administration
The protocol for fluoxetine administration was as follows: Subjects received one capsule per day for 10 weeks, containing placebo for the first 2 weeks, 10 mg fluoxetine for the next week, 20 mg fluoxetine for the following 5 weeks and placebo for the last 2 weeks. They did not know for how long nor at what stage(s) placebo would be administered. They were administered the Hamilton Anxiety and Depression scales and the Comrey Personality Scales and filled in a 10cm Visual Analogue Mood Scale, the Spielberger State Anxiety Inventory, the General Well Being Schedule and the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q). As previously reported, fluoxetine administration had no significant effect on any of these measures (Gelfin et al. 1998).
Challenge Procedure
The subjects underwent ipsapirone challenge on the last or second last day of the initial two week placebo period, before beginning to receive active fluoxetine. A second ipsapirone challenge was performed after 4 weeks of fluoxetine administration Pre-menopausal women were tested during the mid-luteal phase of their menstrual cycle and the entire research protocol was scheduled accordingly. After refraining from food intake for 8 hours, subjects were brought into the test environment (ambient temperature 21 ± 1°C) at 8:30 a.m. on each challenge day. They were at rest in an easy chair throughout the procedure. An indwelling intravenous catheter was inserted into an antecubital vein and kept patent by a heparin lock. At 9:30 a.m., ipsapirone hydrochloride tablets (Tropon, Germany), 0.3 mg/kg, were administered. The dose of ipsapirone was based on that used in our previous study of temperature and hormone responses to ipsapirone in normal subjects (Gelfin et al. 1995). For measurement of plasma ACTH, cortisol, growth hormone and ipsapirone levels, blood samples were collected into pre-chilled tubes containing ethylinediamenetetraacetic acid (EDTA) at −30, 0, 15, 30, 45, 60, 75, 90, 105, 120, 150, and 180 minutes and kept on ice. The samples were centrifuged at 4°C and 1000 g for 15 minutes, within 1 hour of collection, and stored at −80°C until analysis. Sublingual temperature was recorded at −60, −30, 0, 30, 60, 90, 120, 150, and 180 minutes using a high resolution thermistor probe (Hestia, Mannheim, Germany) calibrated between 35.0°C and 42.0°C. Blood pressure and pulse rate, general appearance and subjective effects experienced by the subject were recorded at −30, 0, 30, 60, 120, and 180 minutes. Subjective effects were rated by the subject on a 5 point scale of severity (absent to very severe).
Assays
ACTH and cortisol levels were measured in the Endocrine Laboratory, Department of Medicine, University of Wuerzburg. ACTH was assayed by a sensitive and specific (1–39 ACTH) two-site immunoluminometric assay (Fa. Henning, Berlin, Germany). Intra- and inter-assay coefficients of variation were 3.8% and 9.3%, respectively. Cortisol was measured by radioimmunoassay using commercially available reagents (Biermann GmbH, Bad Nauheim, Germany). Intra- and inter-assay coefficients of variation were 4.4% and 6.4%, respectively.
Plasma growth hormone, ipsapirone and fluoxetine levels were assayed in the University Department of Psychiatry, Warneford Hospital, Oxford. Growth hormone was measured by an in-house immunoradiometric method (IRMA). The international standard IS 80/505 was used as the reference standard. The intra- and inter-assay coefficients of variation (CVs) were both less than 5% over the range of the standard curve (0–100 mIU/L). The assay sensitivity was 0.2 mIU/L. Ipsapirone plasma levels were determined by a high performance liquid chromatographic (HPLC) assay employing reverse phase isocratic HPLC with coulometric end-point detection. The intra and inter-assay coefficients of variation were within 12% and the recovery was generally greater than 75%.
Fluoxetine and it's major metabolite, nor-fluoxetine, were measured in plasma by gas liquid chromatography (GLC) method which utilized a nitrogen/phosphorus specific detector for end-point detection and an internal standard (lignocaine) to monitor extraction recovery. The analytes were extracted from alkalinized plasma (0.5 ml) by a single step solvent (n-heptane with 1.5% iso-amyl alcohol) extraction. The extraction recovery was greater than 80% and the sensitivity was 5 ng/ml. Both the intra- and inter-assay coefficients of variation were within 10%.
For all the assays, all samples from a single subject were measured in duplicate in a single assay run.
Statistical Analysis
Pre- and post-fluoxetine temperature and hormone responses to ipsapirone were compared by analysis of variance (ANOVA) with repeated measures (with Greenhouse Geisser Correction), after normalization of the distribution by logE transformation, if indicated. Initially, the baseline value (recorded immediately prior to administration of the challenge) was entered as a covariate. In the presence of a significant influence of the covariate in the initial analysis, this term was included in the definitive model. Ipsapirone plasma levels were included as a covariate in all analyses of temperature and hormone data. Maximal change in the dependent variable over time was computed by subtracting the baseline from the peak value of each subject. Area under the curve (AUC) was calculated by the trapezoid method. Peak minus baseline (delta) and AUC responses to ipsapirone before and after fluoxetine were compared by ANCOVA with repeated measures with delta or AUC ipsapirone levels as covariate. Bivariate relationships between variables were compared by the Pearson Correlation Coefficient. Differences were accepted as significant on the basis of p < .05 (two tailed). Values in the text and tables are presented as mean ± standard deviation (SD) and in the figures as mean ± standard error of the mean (SEM). Data were analyzed by means of the SAS (release 6.12), BMDP (2V) or SPSS (release 5.0).
RESULTS
Baseline Temperature and Hormone Levels
Table 1 shows the baseline temperature and hormone levels of the 15 subjects on each of the challenge days—after two weeks of placebo (pre-fluoxetine) and after four weeks of fluoxetine administration (post-fluoxetine). No differences were significant at p < .1 by paired t-test (df 14).
Fluoxetine and Ipsapirone Plasma Levels
For technical reasons, data on fluoxetine and ipsapirone levels were not available for one of the subjects. For 14 subjects, the mean (±SD) fluoxetine plasma level after four weeks of administration was 40.97 ± 27.80 ng/ml (range 7.3–110.5 ng/ml, n = 14) and the mean (±SD) norfluoxetine plasma level was 55.25 ± 54.85 ng/ml (range 14.10–193.80 ng/ml).
Figure 1 shows the mean (±SEM) plasma levels of pisapirone after two weeks of placebo administration (pre-fluoxetine) and after 4 weeks of fluoxetine administration (post-fluoxetine). There was a significant increase in plasma ipsapirone levels at the post fluoxetine challenge (Ftreatment = 9.07, df 1,13, p = .01; Ftime = 5.35, df 9,177, p = .01; Finteraction = 1.77, df 9,117, p > .1). As shown in Table 2, both the maximum (p = .03) and the area under the curve ipsapirone levels (p = .01) were significantly higher on fluoxetine (by paired t-tests). To examine whether there was a direct relationship between plasma fluoxetine and norfluoxetine levels (log transformed) and plasma ipsapirone levels, we examined Pearson correlation coefficients. There was no significant correlation between plasma levels of the drug or its metabolite and maximal or AUC ipsapirone levels at the post-fluoxetine challenge (ipsapirone/fluoxetine: maximum, r = .39, AUC, r = .29; ipsapirone/norfluoxetine: maximum, r = .01, AUC, r = .02).
Plasma ipsapirone levels following administration of ipsapirone (0.3 mg/kg) to healthy volunteers receiving placebo (pre-fluoxetine) and after 4 weeks of fluoxetine (post-fluoxetine). Bars represent standard error of the mean. By ANOVA with repeated measures, Ftreatment = 9.07, df 1,13, p = .01; Ftime 5.35, df 9,117, p = .01; Finteraction = 1.77, df 9,117, p > .1.
Temperature Response
Figure 2 shows the mean (±SEM) sublingual temperature recordings over 180 minutes following ipsapirone challenge, after two weeks of placebo administration (pre-fluoxetine) and after four weeks of fluoxetine administration (post-fluoxetine), with baseline temperature subtracted. When the subjects were on fluoxetine, the temperature response to ipsapirone was almost completely blocked (Ftreatment = 5.73, df 1, 12, p = .03; Ftime = 8.16, df 6, 77, p = .0003; Finteraction = 8.65, df 6, 77, p = .0008, by ANCOVA with repeated measures with ipsapirone plasma levels as covariate). As shown in Table 2, both the pre- and post-fluoxetine delta (p = .0005) and AUC (p = .0004) temperature levels reflected this significant effect (by ANOVA with repeated measures with ipsapirone delta or AUC values as covariates).
Change in body temperature in healthy volunteers following administration of ipsapirone (0.3 mg/kg per os) to healthy volunteers receiving placebo (pre-fluoxetine) and after 4 weeks of fluoxetine (post-fluoxetine). (Ftreatment = 5.73, df 1,12, p = .03; Ftime = 8.16, df 6,77, p = .0003; Finteraction = 8.65, df 6,77, p = .0008, by ANCOVA with repeated measures with ipsapirone plasma levels as covariate).
Hormone Responses
ACTH
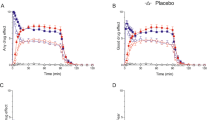
There was a significant effect of fluoxetine to reduce plasma ACTH levels as reflected in the overall time course of hormone levels following the challenge (Figure 3 ; Ftreatment = 10.15, df, 1,11, p = .009; Ftime = 8.16, df 9,116, p = .02; Finteraction = 1.45, df 9,116, p > .1) by ANCOVA with repeated measures with ipsapirone plasma levels and baseline ACTH levels as covariates) and in the delta (p = .04) and AUC values (p = .003) (Table 2).
Plasma ACTH levels (with baseline level subtracted) following administration of ipsapirone (0.3 mg/kg per os) to healthy volunteers receiving placebo (pre-fluoxetine) and after 4 weeks of fluoxetine (post-fluoxetine). (Ftreatment = 10.15, df 1, 11, p = .009; Ftime 8.16, df 9,116, p = .02; Finteraction = 1.45, df 9,116, p > .1; by ANCOVA with repeated measures with ipsapirone plasma levels and baseline ACTH levels as covariates).
Cortisol
As shown in Figure 4 and Table 2, cortisol response to ipsapirone was significantly blunted by fluoxetine administration over the time course of the response (Ftreatment = 10.15, df 1,11, p = .009; Ftime = 8.16, df 9,116, p = .02; Finteraction = 1.45, df 9,116, p > .1; by ANCOVA with repeated measures with ipsapirone plasma levels and baseline cortisol levels as covariates) and when calculated as delta (p =.01) and AUC (p = .002).
Plasma cortisol levels following administration of ipsapirone (0.3 mg/kg per os) to healthy volunteers receiving placebo (pre-fluoxetine) and after 4 weeks of fluoxetine (post-fluoxetine). (Ftreatment = 10.15, df 1,11, p = .009; Ftime = 8.16, df 9,116, p = .02; Finteraction = 1.45, df 9,116, p > .1; by ANCOVA with repeated measures with ipsapirone plasma levels and baseline cortisol levels as covariates).
Growth Hormone
Growth hormone responses to ipsapirone were significantly blunted by fluoxetine when examining the time course of the effect (Figure 5 ; Ftreatment 25.67, df 1,11, p = .0004; Ftime 3.10, df 9,116, p = .04; Finteraction = 1.75, df 9,116, p > .1; by ANCOVA with repeated measures with ipsapirone plasma levels and baseline ACTH levels as covariates) but there was no significant difference between the pre- and post-fluoxetine conditions in the delta (p = .08) or AUC (p = .1) values (Table 2).
Plasma growth hormone levels following administration of ipsapirone (0.3 mg/kg per os) to healthy volunteers receiving placebo (pre-fluoxetine) and after 4 weeks of fluoxetine (post-fluoxetine). (Ftreatment = 25.67, df 1,11, p = .0004; Ftime 3.10, df 9,116, p = .04; Finteraction = 1.75, df 9,116, p > .1; by ANCOVA with repeated measures with ipsapirone plasma levels and baseline growth hormone levels as covariates).
Adverse Effects of Ipsapirone
During the pre-fluoxetine challenge, all but one of the subjects (93.3%) experienced at least one adverse effect following ipsapirone administration. The adverse effects were: dizziness, 13 (86.7%); nausea, 8 (53.3%); weakness, 2 (13.3%); and perspiration, 1 (6.7%). Pallor was observed in 12 (80%) of the subjects. Only in 3 cases was the adverse effect at a level of severity greater than mild. During the post-fluoxetine challenge adverse effects were substantially reduced and were experienced by only 6 (40%) of the subjects (p = .002, Fisher Exact Test). The adverse effects were: dizziness 4 subjects (26.7%), pallor 1 (6.7%), and sleepiness 1 (6.7%). None were at a level of severity greater than mild.
DISCUSSION
Consistent with our hypotheses, we observed blunting of the hypothermic response to the 5-HT1A agonist, ipsapirone, following subchronic administration of fluoxetine to normal volunteers and of the ACTH, cortisol and growth hormone responses to this challenge agent. It should be noted that our study employed 4 weeks of fluoxetine administration, ipsapirone challenge doses that were customized by weight, concomitant assessment of psychological variables and measurement of ipsapirone plasma levels. Our findings extend those of Lesch et al. (1991) by demonstrating the effect of fluoxetine on temperature, ACTH and cortisol responses in normal volunteers, as opposed to patients with OCD, and by observing an effect on growth hormone responses as well. Blunting of hypothermic, cortisol, and growth hormone responses to gepirone were observed by Sargent et al. (1997) in normal volunteers on paroxetine. Anderson et al. (1996) found blunting of the growth hormone response to buspirone in normal volunteers on fluvoxamine. Thus, it can be regarded as well established that SSRIs induce subsensitivity of hypothalamic 5-HT1A receptors in humans after chronic administration, as has been observed by a number of studies in rats (Li et al. 1993, 1994, 1996, 1997). Blunting of the hypothermic response to 5-HT1A agonists can also be regarded as a consistent effect of SSRIs in humans (Lesch et al. 1991; Sargent et al. 1997; present study) and rodents (Goodwin et al. 1985b, 1987b; Maj and Moryl 1992), reflecting subsensitivity of the 5-HT1A receptors which mediate this response.
The location of these receptors has been debated for a considerable time. The initial work of Hjorth (1985) showing that the 5-HT depleting agent p-chlorophenylalanine (PCPA) did not influence the hypothermic effect, suggested a postsynaptic location. Goodwin et al. (1985a, 1987a) showed attenuation of the response in rats and mice both after PCPA and after injection of the serotonergic neurotoxin 5,7-dihydroxytryptamine (5,7-DHT), suggesting a presynaptic location, but it is noteworthy that the attenuation in rats was only seen when measured 14 days after the first PCPA injection and not when measured at 4 days post-PCPA. Evidence for a species difference was provided by Bill et al. (1991), who showed that the response in rats was unaffected by 5,7-DHT and PCPA while that in mice was abolished. Although Hillegaart (1991) obtained a decrease in temperature on direct injection of 8-OH-DPAT into the dorsal raphe in rats, the dose of 8-OH-DPAT used was high and diffusion to postsynaptic sites may have occurred. Several other studies (Hutson et al. 1987; O'Connell et al. 1992; Millan et al. 1993) have now shown that the hypothermic effect in rats is postsynaptically located. Furthermore, although Goodwin et al. (1987b) showed that the hypothermic effect in rats as well as in mice was reduced after electroconvulsive shock (ECS), at least two studies (Blier and Bouchard 1992; Gur et al. 1997) have shown that the sensitivity of the 5-HT1A autoreceptor in the rat dorsal raphe is unaltered by ECS. The only available human data also suggest a postsynaptic location, since Blier et al. (1994) showed no change in the hypothermic response to buspirone in normal human volunteers after tryptophan depletion.
Our findings should be considered in the context of certain limitations of the study. Although we did not include a placebo challenge in the protocol, previous studies (Lesch et al. 1989, 1990b; Gelfin et al. 1995) have clearly shown that ipsapirone significantly increases hypothalamic, ACTH, cortisol and growth hormone responses over the effect of placebo. Moreover, a non-specific action of the challenge to induce secretion of all four hormones and to reduce temperature, is highly unlikely. A second limitation is the absence of a placebo treatment group alongside the subjects receiving fluoxetine. This is needed in order to establish that altered responses to the challenge on treatment are due to a specific action of the SSRI on the 5-HT1A receptor and are not a test-retest phenomenon. In this regard, it should be noted that Sargent et al. (1997) included a placebo group in their study of normal subjects receiving paroxetine and found no change in temperature or hormone responses to gepirone in this group. Moreover, in our study, the first ipsapirone challenge was performed after a two week placebo run-in period which is likely to have compensated, to some extent, for non-specific effects not directly due to the active drug. A third consideration is that fluoxetine significantly increased plasma ipsapirone levels, as was observed by Anderson et al. (1996) for buspirone and Sargent et al. (1997) for gepirone but not by Lesch et al. (1991) in their OCD patients administered fluoxetine. It is unlikely that the effects we observed were due to pharmacokinetic factors since elevated ipsapirone levels would be expected to result in increased rather than decreased temperature and hormone responses. In any event, our statistical analyses took plasma ipsapirone levels into account in all cases (although, it should be noted that the implied assumption of a linear or higher order relationship between hormonal and hypothermic responses and ipsapirone levels may not necessarily be valid).
How do these findings influence our understanding of the role of 5-HT1A receptors in the antidepressant action of SSRIs? If the hypothermic action of ipsapirone is pre-synaptically mediated, a blunted response on fluoxetine is consistent with electrophysiological data showing subsensitivity of somatodendritic autoreceptors following SSRI administration in rodents (de Montigny et al. 1990). Microdialysis findings indicating reduced efficacy of the 5-HT1A autoreceptor to inhibit cell firing in response to 8-OH-DPAT injection (Rutter et al. 1994; Kreiss and Lucki 1995; Invernizzi et al. 1994) are also supported. These pre-synaptic effects would led to increased synaptic availability of 5-HT. At the same time, blunting of ACTH, cortisol and growth hormone release in response to ipsapirone, is indicative of post-synaptic 5-HT1A receptor subsensitivity. This is not consistent with electrophysiological data showing no change in the responsiveness of post-synaptic 5-HT1A receptors after SSRI administration (Blier and de Montigny 1994; Mongeau et al. 1997). The latter finding is referable to the hippocampus while the neuroendocrine responses to ipsapirone are mediated by 5-HT1A receptors in the hypothalamus; regional differences in the effect of SSRIs on 5-HT1A receptor-mediated responses must be considered. However, inhibition of forskolin stimulated adenylate cyclase by 8-OH-DPAT, which is mediated by post-synaptic 5-HT1A receptors, is blunted in the hippocampus after SSRI administration to rats (Newman et al. 1992). This parallels the blunted hormone responses to ipsapirone, mediated by 5-HT1A receptors in the hypothalamus, which we observed. Subsensitive post-synaptic 5-HT1A receptors after SSRIs could be an adaptive response to increased synaptic 5-HT levels. The overall result need not be a reduction in 5-HT neurotransmission since net transmission would depend on the balance between pre-synaptic neuronal firing and post-synaptic adaptation.
An intriguing observation which emerged from our study is that the clinical doses of fluoxetine administered to our subjects had no discernible effect on any of the psychological variables which we examined (Gelfin et al. 1998). These included ratings of mood, anxiety, general well being, various aspects of quality of life and personality characteristics. More subtle effects of paroxetine were observed in a subsequent study of normal volunteers (Knutson et al. 1998)—a reduction in focal indices of hostility through a decrease in negative affect and an increase in a behavior index of social affiliation. These effects were already present after one week of paroxetine administration. The two other studies which examined effects of SSRIs on temperature and neuroendocrine responses in normal subjects did not report effects on mood. (Anderson et al. 1996; Sargent et al. 1997). Since 5-HT1A receptor mediated responses were significantly altered in our subjects while the measures of mood state which we employed, were not (Gelfin et al. 1998), two interpretations are possible. The first is that the significant effects of fluoxetine on 5-HT1A receptor function was observed are epiphenomena and enhanced synaptic availability of 5-HT and changes in 5-HT1A receptor sensitivity do not play a pivotal role in mood and other psychological variables. In this regard, it should be noted that administration of fenfluramine, which acutely increases synaptic levels of 5-HT, did not alter mood in normal subjects in a study previously reported by our group (Lichtenberg et al. 1992). While this interpretation is theoretically feasible, the weight of the evidence supporting a role for 5-HT1A receptors in antidepressant action, whatever the precise mechanisms involved, is difficult to discount. The second possibility is that alterations in 5-HT1A receptor function are functionally relevant to mood when they occur in the clinical (and neurobiological) context of depression but not in normal subjects. Blunted hypothermic (Lesch et al. 1990c, Cowen et al. 1994), ACTH and cortisol (Lesch et al. 1990d; Meltzer and Maes 1994) responsiveness to 5-HT1A receptor agonists, including ipsapirone, have been reported in depressed patients and a reduced hypothermic response to ipsapirone was observed following chronic antidepressant (amitriptyline) administration (Lesch et al. 1990e). Further studies on the effect of SSRIs on 5-HT1A receptor-mediated hypothermic and neuroendocrine responses in depressed patients are needed in order to address this question. These should correlate the effects of the drug on 5-HT1A receptor function and on mood in order to determine whether there is a direct relationship. In parallel, studies encompassing the spectrum of measures used to evaluate 5-HT1A receptor function should be conducted in animal models of depression rather than in “normal” rodents. These should establish whether similar effects on 5-HT1A receptors are observed following SSRI administration and whether there is a relationship between these effects and the action of the drug on behavioral aspects of the model.
References
American Psychiatric Association. (1987): Committee on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC, American Psychiatric Association
Anderson IM, Deakin JFW, Miller HEJ . (1996): The effect of chronic fluvoxamine on hormonal and psychological responses to buspirone in normal volunteers. Psychopharmacology 128: 74–82
Auerbach SB, Hjorth S . (1995): Effect of chronic administration of the selective serotonin uptake inhibitor citalopram on extracellular 5-HT and apparent autoreceptor sensitivity in rat forebrain in vivo. N S Arch Pharmacol 352: 597–606
Bagdy G, Makara GB . (1994): Hypothalamic paraventricular nucleus lesions differentially affect serotonin1A (5-HT1A) and 5-HT2 receptor agonist-induced oxytocin, prolactin, and corticosterone responses. Endocrinology 134: 1127–1131
Berlin I, Warot D, Legout V, Guillement S, Schollnhammer G, Puech AJ . (1998): Blunted 5-HT1A receptor agonist-induced corticotropin and cortisol responses after long term ipsapirone and fluoxetine administration to healthy subjects. Clin Pharmacol Ther 63: 428–436
Bel N, Artigas F . (1993): Chronic treatment with fluvoxamine increases extracellular serotonin in frontal cortex but not in raphe nuclei. Synapse 15: 243–245
Bill DJ, Knight M, Forster EA, Fletcher A . (1991): Direct evidence for a species difference in the mechanism of 8-OH-DPAT-induced hypothermia. Br J Pharmacol 103: 1857–1864
Blier P, Bouchard C . (1992): Effect of repeated electroconvulsive shocks on serotonergic neurons. Eur J Pharmacol 211: 365–373
Blier P, de Montigny C . (1994): Current advances and trends in the treatment of depression. Trends Pharmacol Sci 15: 220–226
Blier P, Seletti B, Young SN, Benkelfat C, de Montigny C . (1994): Serotonin1A receptor activation and hypothermia: Evidence for a postsynaptic mechanism in humans. Neuropsychopharmacology 10 (suppl 35); 92S
Bosker FJ, Klompmakers AA, Westenberg HGM . (1995a): Effects of single and repeated oral administration of fluvoxamine on extracellular serotonin in the median raphe nucleus and dorsal hippocampus of the rat. Neuropharmacology 34: 501–508
Bosker FJ, van Essevelt KE, Klompmakers AA, Westenberg HGM . (1995b): Chronic treatment with fluvoxamine by osmotic minipumps fails to induce persistent functional changes in central 5-HT1A and 5-HT1B receptors, as measured by in vivo microdialysis in dorsal hippocampus of conscious rats. Psychopharmacology 117: 358–363
Chaouloff F . (1993): Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Rev 18: 1–32
Cowen PJ, Power AC, Ware CJ, Anderson IM . (1994): 5-HT1A receptor sensitivity in major depression: A neuroendocrine study with buspirone. Br J Psychiatry 164: 372–379
Cutler NR, Sramek JJ, Keppel-Hesselink JM, Krol A, Roeschen J, Rickels K, Schweizer E . (1993): A double-blind, placebo-controlled study comparing the efficacy and safety of ipsapirone versus lorazepam in patients with generalized anxiety disorder: A prospective multicenter trial. J Clin Psychopharmacol 6: 429–437
de Montigny C, Chaput Y, Blier P . (1990): Modification of serotonergic neuron properties by long-term treatment with serotonin reuptake blockers. J Clin Psychiatr 51 (suppl. B): 4–8
Gelfin Y, Gorfine M, Lerer B . (1998): Effect of clinically equivalent fluoxetine administration on mood and other psychological parameters in healthy volunteers. Am J Psychiatry 155: 290–292
Gelfin Y, Lerer B, Lesch KP, Gorfine M, Allolio B . (1995): Complex effects of age and gender on adrenocorticotrophic hormone and cortisol responses to ipsapirone challenge in normal subjects. Psychopharmacology 120: 356–364
Gilbert F, Brazell C, Tricklebank MD, Stahl SM . (1988): Activation of the 5-HT1A receptor subtype increases rat plasma ACTH concentrations. Eur J Pharmacol 147: 431–437
Goodwin GM, De Souza RJ, Green AR . (1985a): The pharmacology of the hypothermic response in mice to 8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT): A model of pre-synaptic 5-HT1 function. Neuropharmacology 24: 1187–1194
Goodwin GM, De Souza RJ, Green AR . (1985b): Pre-synaptic serotonin receptor-mediated response in mice attenuated by antidepressant drugs and electroconvulsive shock. Nature 317: 531–533
Goodwin GM, De Souza RJ, Green AR, Heal DJ . (1987a): The pharmacology of the behavioral and hypothermic responses of rats to 8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT). Psychopharmacology 91: 506–511
Goodwin GM, De Souza RJ, Green AR . (1987b): Attenuation by electroconvulsive shock and antidepressant drugs of the 5-HT1A receptor mediated hypothermia and serotonin syndrome produced by 8-OH-DPAT in the rat. Psychopharmacology 91: 500–505
Gur E, Lerer B, Newman ME . (1997): Chronic electroconvulsive shock and 5-HT autoreceptor activity in rat brain: an in vivo microdialysis study. J Neural Transm 104: 795–804
Gur E, Lerer B, Newman ME . (1999): Chronic clomipramine and triiodothyronine increase 5-HT levels in rat frontal cortex in vivo: Relationship to 5-HT autoreceptor activity. J Pharmacol Exp Ther 288: 81–87
Haleem DJ, Kennett GA, Whitton PS, Curzon G . (1989): 8-OH-DPAT increases corticosterone but not other 5-HT1A receptor-dependent responses more in females. Eur J Pharmacol 164: 435–443
Hensler JG, Kovachich GB, Frazer A . (1991): A quantitative autoradiographic study of serotonin1A receptor regulation: effect of 5,7-dihydroxytryptamine and antidepressant treatments. Neuropsychopharmacology 4: 131–144
Hillegaart V . (1991): Effects of local application of 5-HT and 8-OH-DPAT into the dorsal and median raphe nuclei on core temperature in the rat. Psychopharmacology 103: 291–296
Hjorth S . (1985): Hypothermia in the rat induced by the potent serotonergic agent 8-OH-DPAT. J Neural Transm 61: 131–135
Hjorth S, Auerbach SB . (1994): Lack of 5-HT1A autoreceptor desensitization following chronic citalopram treatment, as determined by in vivo microdialysis. Neuropharmacology 33: 331–334
Hutson PH, Donohoe TP, Curzon G . (1987): Hypothermia induced by putative 5-HT1A agonist LY 165163 and 8-OH-DPAT is not prevented by 5-HT depletion. Eur J Pharmacol 143: 121–128
Invernizzi R, Bramante M, Samanin R . (1994): Chronic treatment with citalopram facilitates the effect of a challenge dose on cortical serotonin output: Role of pre-synaptic 5-HT1A receptors. Eur J Pharmacol 260: 243–246
Invernizzi R, Bramante M, Samanin R . (1995): Extracellular concentrations of serotonin in the dorsal hippocampus after acute and chronic treatment with citalopram. Brain Res 696: 62–66
Invernizzi R, Bramante M, Samanin R . (1996): Role of 5-HT1A receptors in the effects of acute and chronic fluoxetine on extracellular serotonin in the frontal cortex. Pharmacol Biochem Behav 54: 143–147
Klimek V, Zak-Knapik J, Mackowiak M . (1994): Effects of repeated treatment with fluoxetine and citalopram, 5-HT uptake inhibitors, on 5-HT1A and 5-HT2 receptors in the rat brain. J Psychiatr Neurosci 19: 63–67
Knutson B, Wolkowitz OM, Cole SW, Chan T, Moore EA, Johnson RC, Terpstra J, Turner RA, Reus VI . (1998): Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry 155: 373–379
Koenig JI, Gudelsky GA, Meltzer HY . (1987): Stimulation of corticosterone and β-endorphin secretion in the rat by selective 5-HT receptor subtype activation. Eur J Pharmacol 137: 1–8
Kreiss DS, Lucki I . (1995): Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-HT measured in vivo. J Pharmacol Exp Ther 274: 866–876
Lesch KP, Rupprecht R, Poten B, Muller U, Sohnle K, Fritze J, Schulte HM . (1989): Endocrine responses to 5-hydroxytryptamine1A receptor activation by ipsapirone in humans. Biol Psychiatry 26: 203–205
Lesch KP, Poten B, Soehnle K, Schulte HM . (1990a): Pharmacology of the hypothermic response to 5-HT1A receptor activation in humans. Eur J Clin Pharmacol 39: 17–19
Lesch KP, Soehnle K, Poten B, Rupprecht R, Schulte HM . (1990b): Corticotrophin and cortisol secretion following central 5-hydroxytryptamine1A (5-HT1A) receptor activation: Effects of 5-HT receptor and β-adrenoceptor antagonists. J Clin Endocrinol Metab 70: 670–674
Lesch KP, Mayer S, Disselkamp-Tietze J, Hoh A, Schoellnhammer G, Schulte HM . (1990c): Subsensitivity of the 5-Hydroxytryptamine1A (5-HT1A) receptor-mediated hypothermic response to ipsapirone in unipolar depression. Life Sciences 46: 1271–1277
Lesch KP, Mayer S, Disselkamp-Tietze J, Wiesmann M, Osterheider M, Schulte HM . (1990d): 5-HT1A receptor responsivity in unipolar depression: evaluation of ipsapirone-induced ACTH and cortisol secretion in patients and controls. Biol Psychiatry 28: 620–628
Lesch KP, Disselkamp-Tietze J, Schmidtke A . (1990e): 5-HT1A receptor function in unipolar depression: Effect of chronic amitriptyline treatment. J Neural Transm 180: 157–161
Lesch KP, Hoh A, Schulte HM, Osterheider M, Muller T . (1991): Long-term fluoxetine treatment decreases 5-HT1A receptor responsivity in obsessive-compulsive disorder. Psychopharmacology 105: 415–420
Li Q, Levy AD, Cabrera TM, Brownfield MS, Battaglia G, van der Kar L . (1993): Long-term fluoxetine, but not desipramine, inhibits the ACTH and oxytocin responses to the 5-HT1A agonist, 8-OH-DPAT, in male rats. Brain Res 630: 148–156
Li Q, Brownfield MS, Levy AD, Battaglia G, Cabrera TM, van de Kar L . (1994): Attenuation of hormone responses to the 5-HT1A agonist, ipsapirone, by long-term treatment with fluoxetine, but not desipramine, in male rats. Biol Psychiat 36: 300–308
Li Q, Muma NA, van der Kar L . (1996): Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and Go proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharmacol Exp Ther 279: 1035–1042
Li Q, Muma NA, Battaglia G, van der Kar L . (1997): A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: Reduction in the levels of Gi and Go proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther 282: 1581–1590
Lichtenberg P, Shapira B, Blacker M, Gropp C, Calev A, Lerer B . (1992): Effect of fenfluramine on mood: A double-blind, placebo-controlled trial. Biol Psychiatry 31: 351–356
Maj J, Bijak M, Dziedzicka-Wasylewska M, Rogoz R, Rogoz Z, Skuza G, Tokarski T . (1996): The effects of paroxetine given repeatedly on the 5-HT receptor subpopulations in the rat brain. Psychopharmacology 127: 73–82
Maj J, Moryl E . (1992): Effect of sertraline and citalopram given repeatedly on the responsiveness of 5-HT receptor subpopulations. J Neural Transm 88: 143–156
Meltzer HY, Bastani B, Jayathilake K, Maes M . (1997): Fluoxetine, but not tricyclic antidepressants, potentiates the 5-hydroxytryptophan-mediated increase in plasma cortisol and prolactin secretion in subjects with major depression or with obsessive compulsive disorder. Neuropsychopharmacology 17: 1–11
Meltzer HY, Maes M . (1994): Effect of pindolol on the L-5-HTP-induced increase in plasma prolactin and cortisol concentrations in man. Psychopharmacology 114: 635–643
Meltzer HY, Maes M . (1995): Effects of ipsapirone on plasma cortisol and body temperature in major depression. Biol Psychiatry 38: 450–457
Meltzer HY, Wiita B, Robertson A, Tricou BJ, Lowy M, Perline R . (1984): Effect of 5-hydroxytryptophan on serum cortisol levels in major affective disorders: Enhanced response in depression and mania. Arch Gen Psychiatr 41: 366–374
Millan MJ, Rivet J-M, Canton H, LeMarouille-Girardon S, Gobert A . (1993): Induction of hypothermia as a model of 5-hydroxytryptamine1A receptor-mediated activity in the rat: A pharmacological characterization of the actions of novel agonists and antagonists. J Pharmacol Exp Ther 264: 1364–1376
Mongeau R, Blier P, de Montigny C . (1997): The serotonergic and noradrenergic systems of the hippocampus: Their interactions and the effects of antidepressant treatments. Brain Res Review 23: 145–195
Newman ME, Drummer D, Lerer B . (1990): Single and combined effects of desipramine and lithium on serotonergic receptor number and second messenger function in rat brain. J Pharmacol Exp Ther 252: 826–831
Newman ME, Lerer B . (1988): Chronic electroconvulsive shock and desipramine reduce the degree of inhibition of 5 HT and carbachol of forskolin stimulated adenylate cyclase in rat hippocampal membranes. Europ J Pharmacol 148: 257–260
Newman ME, Shapira B, Lerer B . (1992): Regulation of 5-HT1A receptor function in rat hippocampus by short- and long-term administration of 5-HT1A agonists and antidepressants. J Pharmacol Exp Ther 260: 16–20
O'Connell MT, Sarna G, Curzon G . (1992): Evidence for postsynaptic mediation of the hypothermic effect of 5-HT1A receptor activation. Br J Pharmacol 106: 603–609
O'Donnell JM, Grealy M . (1992): Neuroendocrine response to clonidine and 8-OH-DPAT in rats following chronic administration of desipramine or sertraline. Br J Pharmacol 105: 863–868
Peroutka SJ . (1988): 5-Hydroxytryptamine receptor subtypes: molecular, biochemical and physiological characterization. Trends Neurosci 11: 496–500
Przegalinski E, Warchol-Kania A, Budziszewska B . (1989): The lack of effect of repeated treatment with antidepressant drugs on the 8-OH-DPAT-induced increase in the serum corticosterone concentration. Pol J Pharmacol Pharm 41: 63–68
Rutter JJ, Gundlah C, Auerbach SB . (1994): Increase in extracellular serotonin produced by uptake inhibitors is enhanced after chronic treatment with fluoxetine. Neurosci Lett 171: 183–186
Sargent P, Williamson DJ, Pearson G, Odontiadis J, Cowen PH . (1997): Effect of paroxetine and nefazodone on 5-HT1A receptor sensitivity. Psychopharmacology 132: 296–302
Spitzer RL, Endicott J . (1978): Schedule for Affective Disorders and Schizophrenia—Lifetime Version (SADS-L). New York, New York State Psychiatric Institute
Tanda G, Frau R, di Chiara G . (1996): Chronic desipramine and fluoxetine differentially affect extracellular dopamine in the rat prefrontal cortex. Psychopharmacology 127: 83–87
Traber J, Glaser T . (1987): 5-HT1A receptor-related anxiolytics. Trends Pharmacol Sci 8: 432–437
Welner SA, de Montigny C, desRoches J, desJardins P, Suranyi-Cadotte BE . (1989): Autoradiographic quanitification of serotonin1A receptors in rat brain following antidepressant drug treatment. Synapse 4: 347–352
Acknowledgements
The authors thank Dr. Michael Franklin, University Department of Psychiatry, Warneford Hospital, Oxford for measurement of plasma growth hormone, ipsapirone, and fluoxetine levels. Ipsapirone tablets were supplied by Tropon, Bayer.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lerer, B., Gelfin, Y., Gorfine, M. et al. 5-HT1A Receptor Function in Normal Subjects on Clinical Doses of Fluoxetine: Blunted Temperature and Hormone Responses to Ipsapirone Challenge. Neuropsychopharmacol 20, 628–639 (1999). https://doi.org/10.1016/S0893-133X(98)00106-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(98)00106-7
Keywords
This article is cited by
-
Slow-release delivery enhances the pharmacological properties of oral 5-hydroxytryptophan: mouse proof-of-concept
Neuropsychopharmacology (2019)
-
Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice
Molecular Psychiatry (2012)
-
Pharmacology of ayahuasca administered in two repeated doses
Psychopharmacology (2012)
-
5HT1A-mediated stimulation of cortisol release in major depression: use of non-invasive cortisol measurements to predict clinical response
European Archives of Psychiatry and Clinical Neuroscience (2010)
-
Influence of escitalopram treatment on 5-HT1A receptor binding in limbic regions in patients with anxiety disorders
Molecular Psychiatry (2009)