Abstract
Fifteen smokers participated in a study investigating brain correlates of nicotine dependence. Dependence was reduced by having subjects switch to denicotinized cigarettes for 2 weeks while wearing nicotine skin patches. Positron emission tomography (PET) scans assessed regional cerebral metabolic rate for glucose (rCMRglc) after overnight nicotine abstinence on three occasions: (1) at baseline; (2) after 2 weeks of exposure to denicotinized cigarettes+nicotine patches; and (3) 2 weeks after returning to smoking the usual brands of cigarettes. Craving for cigarettes and scores on the Fagerström Test of Nicotine Dependence (FTND) questionnaire decreased at the second session relative to the first and last sessions. Regional brain metabolic activity (normalized to whole brain values) at session 2 also showed a significant decrease in the right hemisphere anterior cingulate cortex. Exploratory post hoc analyses showed that the change in craving across sessions was negatively correlated with the change in rCMRglc in several structures within the brain reward system, including the ventral striatum, orbitofrontal cortex and pons. The between-session difference in thalamus activity (right hemisphere) was positively correlated with the difference in FTND scores. Correlational analyses also revealed that reported smoking for calming effects was associated with a decrease (at session 2) in thalamus activity (bilaterally) and with an increase in amygdala activity (left hemisphere). Reported smoking to enhance pleasurable relaxation was associated with an increase in metabolic activity of the dorsal striatum (caudate, putamen) at session 2. These findings suggest that reversible changes in regional brain metabolic activity occur in conjunction with alterations in nicotine dependence. The results also highlight the likely role of thalamic gating processes as well as striatal reward and corticolimbic regulatory pathways in the maintenance of cigarette addiction.
Similar content being viewed by others
INTRODUCTION
Recent decades have witnessed a rapid expansion in our knowledge about brain mechanisms underlying drug addiction (Kalivas and Volkow, 2005; Koob and Le Moal, 2005; Hyman et al, 2006). The nervous system pathways and neurotransmitters mediating drug reinforcement have been studied from a variety of perspectives, including animal models and human behavioral pharmacology investigations. These models have been applied to the study of tobacco addiction, which remains a very serious public health problem. A great deal of evidence has implicated nicotine in the acquisition and maintenance of tobacco addiction (U.S.D.H.H.S., 1988); nonetheless, the mechanisms underlying human cigarette dependence have yet to be fully elucidated. An understanding of brain mechanisms underlying addiction may lead to the development of more effective smoking cessation therapies by identifying new targets for medication development, and by highlighting individual differences that may require tailored interventions.
A potentially useful approach for this investigation in human smokers is the application of relatively noninvasive brain imaging techniques, including positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), to study the brain pathways involved in dependence on nicotine and craving for cigarettes. Several studies have reported that nicotine acutely affects brain activity as indexed by global or regional cerebral metabolic rate for glucose (rCMRglc) (Domino et al, 2000; Stapleton et al, 2003). For example, in a study on the effect of nicotine on rCMRglc in human volunteers, using the [F-18]-fluorodeoxyglucose (FDG) method and PET (Stapleton et al, 2003), five subjects participated in two assessments of the effects of 1.5 mg intravenous (i.v.) nicotine and produced a generalized global reduction of about 10% of the cerebral glucose metabolism assayed in a saline control condition. Domino et al (2000) examined the effects of nicotine (administered via nasal spray) on cerebral glucose metabolism, and also found a trend for a global reduction in CMRglc, with relative regional increases observed in thalamus and occipital cortex.
Another approach to assess central nervous system actions of nicotine is by the use of regional cerebral blood flow (rCBF) measurements, using [O-15]-labeled water and PET (Fox et al, 1986). Diverse effects have been reported (Mathew and Wilson, 1995), including increases, decreases (Cruikshank et al, 1989), or no effect (Solti et al, 1963). However, most studies have reported localized increases in rCBF after nicotine administration (eg, Skinhoj et al, 1973; Wennmalm, 1982). Nagata et al (1995), for example, reported significant increases in rCBF after cigarette smoking, most notably in the frontal lobes and cerebellum. Stein et al (1998), using fMRI to measure rCBF after i.v. nicotine administration, found activation in several brain regions involved in reinforcement, including nucleus accumbens, amygdala, anterior cingulate cortex, and frontal lobes. Zubieta et al (2001) reported that intranasal nicotine administration increased blood flow in the right hemisphere thalamus, but decreased blood flow in the left anterior temporal cortex and right hemisphere amygdala. A similar result was found in a study by Rose et al (2003), administering cigarette smoke with an individualized dosing to match subjects' habitual smoking pattern. Administration of nicotine-containing cigarettes vs denicotinized cigarettes produced a dose-related decrease in blood flow in the amygdala as well as in the occipital cortex and reticular activating system (thalamus, midbrain, and pons); decreased activity in this system correlated with the extent of craving reduction by nicotine. The nicotinic antagonist mecamylamine generally had opposite effects to those of nicotine. In summary, the literature on the effects of nicotine on limbic regions is mixed; whereas nicotine administration sometimes increases regional brain activity assessed by rCBF, in other studies nicotine withdrawal is associated with higher levels of activity.
The functional effects of nicotine or smoking on brain activity while subjects were engaged in cognitive tasks have also been investigated in several studies. Acute effects of cigarette smoking on rCBF during performance of an auditory task were reported by Flaum et al (1994); postsmoking increases were detected in brainstem (pons), occipital cortex, and right medial temporal/insular cortex. Ghatan et al (1998) found that i.v. administration of nicotine decreased rCBF in the anterior cingulate cortex and cerebellum, and increased rCBF in the occipital cortex during an attention-demanding psychometric task. Ernst et al (2001) similarly found that nicotine administration (polacrilex gum) reduced task-related activation in the anterior cingulate cortex. Thus, overall, nicotine administration appears to decrease activity of the anterior cingulate cortex relative to withdrawal during cognitive task performance.
Although several studies have investigated the acute effects of nicotine, very few have attempted to explore changes in brain activity accompanying chronic smoking or responses to smoking cessation treatment medications. One such study that explored the relationship between craving and brain activity after smoking cessation treatment was conducted by Brody et al (2004), who reported that after treatment with bupropion, cue-elicited craving diminished in proportion to decreased activation of the orbitofrontal cortex. The investigators also measured decreased activation of the anterior cingulate cortex after treatment. However, in this study, cigarette consumption covaried with treatment condition, and therefore the study could not rule out direct effects of bupropion or changes in nicotine levels accompanying treatment.
In the present experiment, we manipulated smokers' level of nicotine dependence and craving for cigarettes, while holding constant nicotine levels during the brain-imaging sessions. Sessions were always conducted in a nicotine-deprived state, after overnight abstinence. Smokers' dependence level was altered by smoking denicotinized cigarettes for 2 weeks, in conjunction with nicotine patch treatment, a procedure that we have found to reduce measures of nicotine dependence (Rose et al, 2006). We sought to determine whether this selective manipulation of dependence on cigarettes would cause definable changes in brain function that could be assessed using PET. If so, the knowledge might yield new clues about brain mechanisms underlying addiction to cigarettes. Based on the finding in several previous studies that anterior cingulate cortex activity was increased during nicotine withdrawal or while craving cigarettes, one specific hypothesis was that a reduction in dependence on cigarettes would decrease anterior cingulate metabolic activity assessed during nicotine withdrawal. It was less clear what effects would be observed in other limbic or cortical regions (eg, thalamus, striatum, prefrontal cortex), because in these regions nicotine withdrawal has often been associated with reduced activity. However, cue-induced craving generally has an opposite effect, that is an increase in activity. Thus reducing the degree of dependence on cigarettes, with a concomitant decrease in craving, might be expected to either increase or decrease activity in these regions.
METHODS
Subjects
Fifteen smokers with no known serious health problems were recruited from the community by advertisements. To be included in the study, potential subjects had to be 21–60 years of age, have an estimated daily nicotine intake from cigarettes of at least 10 mg (calculated by multiplying the number of cigarettes smoked daily by the rated nicotine yield of each brand according to the Federal Trade Commission's determinations (Federal Trade Commission, 2000)), have afternoon end-expired air carbon monoxide (CO) concentrations of at least 15 p.p.m. (confirming they were inhaling smokers), and score ⩾6 on the Fagerström Test of Nicotine Dependence (FTND) questionnaire (Heatherton et al, 1991), indicating at least moderately high dependence. Subjects were further screened based on physical examination, electrocardiogram, serum chemistries, complete blood count, and urinalysis (including a urine screen for drugs of abuse). Anyone diagnosed with a major medical condition (including hypertension, coronary artery disease, cardiac rhythm disorders, current psychiatric illness, pregnancy, or nursing mothers) was excluded from participation. Current alcohol or other non-nicotine drug abuse, smokeless tobacco use, use of nicotine replacement therapy (NRT), or other smoking cessation treatment was also a basis for exclusion.
To facilitate subject recruitment, volunteers were offered an incentive of $150 per session for PET imaging and $50 for an MRI session (this MRI scan was used to determine anatomical regions of interest (ROIs) for subsequent PET analysis). Subjects were also informed about ongoing smoking cessation treatment trials in our program.
Design and Procedures
We measured rCBF and rCMRglc after overnight deprivation from cigarettes (nicotine patches were also removed the night before scanning sessions), at three time points: (1) at baseline, before being switched to denicotinized cigarettes (‘Next’ and ‘Quest 3’ brand cigarettes were used: Federal Trade Commission (FTC) nicotine yields were less than 0.1 mg, ‘tar’ yield 9–10 mg); (2) after 2 weeks of smoking denicotinized cigarettes and using nicotine skin patches (21 mg/24 h); and (3) 2 weeks after resuming smoking of the usual brands of cigarettes (see Figure 1).
Indices of Smoking Behavior
Daily diary counts of cigarettes smoked, plasma cotinine, and expired air CO measurements taken at each session were used to assess smoking behavior during the weeks between sessions. Plasma nicotine levels at the beginning of study sessions were used to confirm compliance with removal of nicotine patches the previous evening.
Questionnaire Assessments
The FTND questionnaire for assessing the degree of nicotine dependence was filled out at the beginning of each session; at sessions 2 and 3, subjects answered questions with reference to the preceding weeks since the last assessment. Smoking withdrawal symptoms were also assessed at each session using a modified version of the Shiffman–Jarvik questionnaire (Shiffman and Jarvik, 1976). Scales included craving, missing the habit aspects of smoking, dysphoria (negative effect), stimulation, appetite, somatic symptoms (separate clusters for anxiety, gastrointestinal, and respiratory symptoms).
Two questionnaires to assess self-reported motivations for smoking were also administered. One questionnaire, an abbreviated version of a smoking motivation questionnaire developed by Russell et al (1974), classified smoking-related situations and motives into ‘stimulation,’ ‘relaxation,’ ‘craving,’ ‘psychosocial,’ ‘handling,’ ‘crutch,’ and ‘automatic’ smoking. Each of 20 items was answered using a numerical rating scale ranging from 1 (‘not at all’) to 4 (‘very much so’). Because this questionnaire does not clearly distinguish between situations that elicit smoking and the anticipated consequences of smoking, we administered a second questionnaire (‘Reasons to Smoke Questionnaire’) that specifically asked subjects to rate (using numerical scales ranging from 1 (‘least important’) to 7 (‘most important’)) the importance of smoking to obtain the following rewarding effects: ‘It calms me down,’ ‘It gives me something to do with the hands,’ ‘I like the taste and smell,’ ‘I like the sensations deep in my throat or chest,’ ‘It wakes me up when I am drowsy,’ ‘I like to watch the smoke,’ It makes relaxing seem even better,’ ‘It satisfies my craving,’ ‘It gives me a rush,’ ‘It gives me more confidence around people,’ ‘It is like a friend,’ and ‘It helps me concentrate.’
Continuous Performance Task
To standardize the behavioral environment during the FDG uptake period used for brain metabolic rate measurements, as well as to assess the effects of the nicotine dependence manipulation on cognitive performance, a continuous performance test (Mayes and Calhoun, 1999) was administered during each session (Gordon Systems Inc., DeWitt, NY). For three 15-min segments, subjects listened to a sequence of digits presented aloud from loudspeakers at a rate of approximately 1/s, and were instructed to depress a button when the number ‘9’ was immediately preceded by a ‘1.’ This task is similar to a ‘1-back’ task (Xu et al, 2005). The task was computer scored in terms of number of errors of omission and errors of commission.
General PET and MRI Methods
All PET imaging was performed on a General Electric Medical Systems Advance scanner (Milwaukee, WI) (DeGrado et al, 1994); measures of brain activity included relative rCBF, assessed with the [O-15] water method, and measures of regional neuronal glucose metabolism (rCMRglc), assessed with FDG. The rCBF assessment was conducted before the cognitive task and the rCMRglc assessment occurred during task performance. Corrections were made for random events, scatter, dead time, detector normalization, and attenuation (see below) before reconstruction via filtered back-projection. Images were reconstructed into 128 × 128 × 35 transaxial image sets with 2 mm pixels in 4.25 mm contiguous slices. Each subject's head was aligned in a plane roughly parallel to the glabella–inion (G–I) line. A transmission scan was performed before or after each emission scan for the purpose of attenuation correction. The specific protocols for assessing metabolic rate and blood flow are described as follows.
CBF imaging protocol
To measure rCBF with PET, we implemented the 15O-water technique as described by Madden et al (2002). After an i.v. bolus injection of 12 mCi of 15O-water, each three-dimensional PET measurement acquisition began when a total count rate of 100 k/s was reached, and continued for 60 s.
CMRglc imaging protocol
Quantitative cerebral metabolic rate was measured with FDG. Subjects received an FDG injection (10 mCi dose) followed by a 30 min uptake period. Subsequently a 10 min emission brain scan was performed. rCMRglc images were determined with the method of Phelps et al (1979). Input functions were determined from dynamic scanned images of the cardiac blood pool before and after the static image of the brain. In this method, arterial input functions are determined from images acquired of the left ventricle (LV) blood pool. The volunteer was positioned in the scanner for a scan of the heart (confirmed by a transmission scan) at the time of injection. At the injection time, a dynamic series of acquisitions commenced, lasting 30 min. This was followed by the brain scan (after the appropriate scanner bed motion), and a final static image of the heart was acquired after the brain scan to complete the arterial input function curve. The arterial radioactivity concentration was determined by ROI analysis of the LV. During the earliest frames, the LV blood pool radioactivity peaks, and is considerably higher than surrounding tissue. The image set from such a frame was used to determine a blood pool ROI. This multislice ROI was as large as possible, while being sufficiently conservative to avoid partial volume loss and spillover from the myocardium. The ROI was applied to all frames of the thorax data to determine the entire input function curve (DeGrado et al, 1996).
MR imaging
Transaxial images were acquired with a 1.5 T scanner (Signa GE Medical Systems, Milwaukee, WI). Both T2-weighted [2500/20;80; repetition time (TR) (ms)/echo time (TE) (ms)] (1 Nex) and T1-weighted [600/20; TR (ms)/TE (ms)] (2 Nex) images were acquired with a 128 × 256 matrix with a 24 cm field of view. Contiguous 3 mm slices were acquired for coregistration with the PET images.
Image registration
We registered the PET image for each subject to his/her own MRI, following methods of Pelizzari et al (1989) with modifications (Turkington et al, 1995). The image registration allows re-orienting of the PET images by re-slicing to match the finer-sampled MR images within 1–2 mm accuracy so that MR-identified brain regions can be applied to the PET data. T1-weighted images were used for the determination of ROI. ROI evaluation started with the sampling of brain regions on each subject's MR image. A trained operator drew regions that included the appropriate gray matter, incorporating local thresholds to differentiate gray and white matter.
Control of prescan state
Subjects were asked to avoid alcohol and nicotine for 12 h before the session and calorie-containing foods after midnight. Caffeine intake before the session was not restricted, to avoid caffeine-withdrawal symptoms (eg, headache). After insertion of the i.v. line and positioning the subject in the scanner, a mock PET scan was performed without radiopharmaceutical administration to facilitate habituation to the procedure.
Data Analysis
Dependent variables were analyzed by repeated-measures analysis of variance (Superanova and Statview, Inc., Cary, NC), using a general linear modeling approach. Given the A-B-A nature of the design, effects of the treatment with denicotinized cigarettes+nicotine patches were assessed by conducting contrasts that compared measures at session 2 (after 2 weeks of smoking denicotinized cigarettes and wearing nicotine patches) to the average of session 1 (baseline) and session 3 values (after 2 weeks of smoking usual brand cigarettes).
The absolute brain metabolic rate for glucose was calculated for each entire hemisphere, whereas for each subregion of interest, rCMRglc values were normalized to whole brain (average of left and right hemispheres). For each hemisphere, the following ROIs were analyzed: anterior cingulate cortex, prefrontal cortex (subsection of the frontal lobe, superior to the orbital gyri, at the axial level of the anterior cingulate, extending approximately 30° from the midline), orbitofrontal cortex, temporal cortex, amygdala, thalamus, caudate, putamen, midbrain, colliculi (superior and inferior), ventral striatum (posterior aspect of the basal ganglia, where the boundary between the caudate and putamen could not be defined), and pons. Many of these regions were chosen because of having been identified as responsive to nicotine or smoking manipulations in previous studies (eg, Brody et al, 2002, 2004). Several of the regions are integral parts of mesolimbic brain reward pathways (eg, ventral striatum, anterior cingulate cortex, orbitofrontal cortex), which have been found to respond to administration of psychoactive drugs, including nicotine (eg, Stein et al, 1998; Breiter and Rosen, 1999; Rose et al, 2003; Domino et al, 2004). The anterior cingulate cortex, in particular, has received considerable attention in studies of cue-elicited craving (Brody et al, 2004). Some regions also contain high densities of nicotinic receptors (eg, thalamus, superior and inferior colliculus; Clarke, 1990; Tribollet et al, 2004).
Measures of smoking behavior, withdrawal symptoms, dependence (FTND questionnaire), and task performance were similarly analyzed by contrasting session 2 with sessions 1 and 3.
In addition to the primary outcome analyses above, a number of correlational analyses were also conducted to gain clues about which brain regions might underlie various aspects of nicotine dependence as well as individual subject characteristics. Correlations between the between-session difference in rCMRglc and corresponding differences in the following variables were computed: nicotine dependence (FTND) score, craving (and other withdrawal symptom subscales), and task performance (commission and omission errors). In calculating these correlations, the differences between session 2 values and the average of session 1 and session 3 values were used. The change in rCMRglc as well as the average rCMRglc values (across all sessions) were also correlated with a number of other individual subject characteristics, including age, gender, compliance with instructions to smoke only denicotinized cigarettes during the 2-week treatment period (assessed by diaries reporting the number of other cigarettes smoked daily), and scores on items or subscales of smoking motivation questionnaires administered at baseline. These analyses were viewed as exploratory for the purpose of hypothesis generation.
A total of approximately 750 correlations were computed, raising the issue of type I error control. A Bonferroni-type adjustment of the α criterion in this situation would, we felt, lead to an overly stringent approach, requiring a p<0.0001, thus making it difficult to detect even powerful relationships between variables. We instead opted to assess the results by examining coherent patterns in the data, regarding the analyses as exploratory, and retaining the usual 0.05 α criterion for statistical significance. While approximately 37 significant correlations at p<0.05 would be expected to occur by chance (1/20 on the assumption of independence, fewer if not independent), 52 actually met this criterion; seven chance associations would have been expected at p<0.01, whereas 14 were observed, and less than one would be expected at p<0.001, whereas five were found. This outcome suggests that many of these correlations, and especially the stronger relationships as well as those that comprise integrated patterns, are likely to be meaningful results, at least in terms of hypothesis generation for future validation studies.
Thus, statistical significance was assessed using a two-tailed α=0.05; if results were significant for one hemisphere, trends (p⩽0.1) are reported for the other hemisphere. Unilateral effects are followed up with an analysis to evaluate the significance of the interaction with hemisphere. Data points were evaluated as potential outliers and eliminated if they exceeded four standard deviations from the mean (excluding the value in question), as described by Van Selst and Jolicouer (1994). Brain imaging data from one participant were not analyzed because of the unavailability of a structural MRI scan for defining ROIs.
In a final analysis, the correlations between rCMRglc and normalized rCBF were computed for each brain region of interest, to determine whether rCBF, an indirect marker of neuronal activation, captured similar information to that obtained from brain metabolic measurements.
RESULTS
Subject Characteristics
The sample was 60% female (nine women, six men) and 67% Caucasian (10 Caucasians, five African-Americans). Subjects smoked an average of 23.0 cigarettes/day (SD=8.5) having a mean FTC-rated nicotine yield of 0.8 mg (SD=+0.3). Mean age was 36.7 years (SD=13.5) and subjects had smoked on average for 19.3 years (SD=14.1). Mean FTND score was 6.9 (SD=1.4).
Effects of Treatment on Nicotine Dependence, Withdrawal Symptoms, Smoking Behavior and Task Performance
We hypothesized, based on previous studies, that 2 weeks' exposure to denicotinized cigarettes+nicotine patch treatment would reduce indices of cigarette smoking and nicotine dependence. Indeed, the number of cigarettes smoked daily, expired air CO, and plasma cotinine levels declined markedly at session 2. Cotinine levels averaged 326 ng/ml (SD=202.1) at sessions 1 and 3 vs 188 ng/ml (SD=123.1) at session 2; F(1,14)=8.21, p=0.01 for the contrast. Expired air CO averaged 11.8 p.p.m. (SD=7.0) at sessions 1 and 3 vs 8.5 p.p.m. (SD=5.0) at session 2; F(1,28)=11.93, p=0.002. Cigarettes/day averaged 22 cigarettes/day (SD=7.0) at sessions 1 and 3 vs 18 cigarettes/day (SD=9) at session 2; F(1,24)=8.42, p=0.008. FTND score averaged 6.8 (SD=1.5) at sessions 1 and 3 vs 6.4 (SD=1.8) at session 2; F(1,28)=3.78, p=0.06.
Craving for cigarettes was also less severe at session 2. Craving ratings averaged 5.9 (SD=1.0) at sessions 1 and 3 vs 5.2 (SD=1.8) at session 2; F(1,28)=5.07, p=0.03. No significant differences were noted for other withdrawal symptoms, and there were no significant differences in task performance at session 2 in comparison with sessions 1 and 3.
Effects of Treatment on Regional Brain Activity
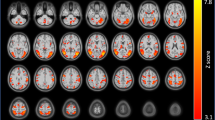
Based on previous studies (Brody et al, 2004; Ernst et al, 2001; Ghatan et al, 1998), we hypothesized that anterior cingulate cortex activity would decrease in session 2, when subjects were less dependent on cigarettes and their withdrawal symptoms less severe. Analysis of normalized rCMRglc did show a significant effect of treatment on activity of the right hemisphere anterior cingulate cortex, F(1,26)=11.44, p=0.002 for the comparison of sessions 1 and 3 against session 2. No effect was seen in the left hemisphere (p>0.3), and the interaction of hemisphere by session contrast was significant (F(1,26)=5.70, p=0.02). Moreover, subjects (n=3) who reported smoking no cigarettes other than the denicotinized cigarettes dispensed to them during the 2 weeks of treatment showed a significantly greater decrease in right hemisphere rCMRglc than subjects showing only partial compliance, as shown in Figure 2; F(1,11)=5.49, p=0.04. Within the high compliance group, the decrease in metabolic activity at session 2 was highly significant (F(1,4)=178.44, p=0.0002).
Effects of the 2-week treatment with denicotinized cigarettes+nicotine patches (and return to usual brand cigarettes) on right hemisphere anterior cingulate rCMRglc, shown separately for subjects who reported complete compliance with smoking denicotinized cigarettes and other subjects reporting incomplete compliance.
The analysis of absolute rCMRglc for left and right hemispheres showed no significant changes across sessions.
Correlations between rCMRglc and Nicotine Dependence/Withdrawal Symptoms
Although a main effect of treatment was not observed in some brain regions thought to be important in nicotine dependence (eg, ventral striatum), exploratory correlational analyses were conducted to seek potential relationships between regional brain activity and dependence/withdrawal symptoms. Given that nicotine activates the mesolimbic reward system and nicotine withdrawal can impair reward function (Koob and Le Moal, 2005), an increase in activity of the ventral striatum and related structures might have been predicted to accompany the decrease in symptoms of dependence at session 2. On the other hand, the positive correlation often observed between cue-induced craving and striatal activity (eg, David et al, 2005) would predict that, to the extent dependence and craving decreased, striatal activity might show a corresponding decrease. For each of the correlations listed below, the difference between the mean of session 1 and 3 values (after exposure to high-nicotine cigarettes) and session 2 values (after smoking denicotinized cigarettes for 2 weeks) for a given rating scale was correlated with the corresponding difference in regional brain metabolic activity. Thus, a positive correlation indicated that as session 1 and 3 values on subjective rating scales were higher than session 2 values, so too were the differences between metabolic activity for a given region.
To summarize the correlations discussed below, metabolic activity of the thalamus tended to be positively associated with nicotine dependence, missing the smoking habit and with somatic anxiety symptoms. Metabolic activity of the ventral striatum tended to be negatively correlated with withdrawal symptoms, including craving, negative affect, stimulation, and somatic anxiety symptoms. Similarly, metabolic activity of the orbitofrontal cortex was negatively correlated with craving and stimulation, and activity in the pons was negatively correlated with dysphoria.
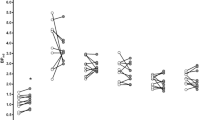
Specifically, the difference in nicotine dependence (FTND) scores between sessions was positively correlated with the corresponding difference in metabolic activity of the thalamus (significant for right hemisphere only; r=0.64, p=0.01; hemisphere interaction NS) (see Figure 3). The between-session difference in ratings of missing the smoking habit was also positively correlated with differences in metabolic activity of the thalamus (significant for left hemisphere only; r=0.56, p=0.04; F(1,12)=6.74, p=0.02 for the hemisphere interaction).
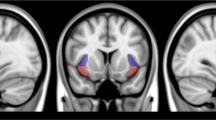
The difference in craving between sessions was negatively correlated with the corresponding difference in metabolic activity of ventral striatum (r=−0.54, p=0.04 for the left hemisphere; r=−0.6, p=0.02 for the right hemisphere) and orbitofrontal cortex (r=−0.64, p=0.01 for the left hemisphere; r=−0.54, p=0.04 for the right hemisphere) (see Figures 4 and 5).
The difference in dysphoria (negative affect symptoms) between sessions was negatively correlated with the difference in activity of ventral striatum (r=−0.57, p=0.03 for the left hemisphere; r=−0.51, p=0.06 for the right hemisphere) and pons (significant for right hemisphere only; r=−0.55, p=0.04; hemisphere interaction NS).
The difference in ratings of stimulation between sessions was negatively correlated with the difference in activity of ventral striatum (significant for right hemisphere only; r=−0.68, p=0.007; hemisphere interaction NS) and orbitofrontal cortex (r=−0.66, p=0.01 for the left hemisphere; r=−0.48, p=0.07 for the right hemisphere).
The difference in ratings of somatic anxiety symptoms between sessions was positively associated with differences in thalamic metabolic activity (significant for right hemisphere only; r=0.57, p=0.03; hemisphere interaction NS) and negatively correlated with activity of the ventral striatum (significant for right hemisphere only; r=−0.57, p=0.03; hemisphere interaction NS).
Correlations between rCMRglc and Smoking Motivation
We hypothesized that correlating regional brain activity with self-reported motives for smoking might reveal associations with brain function not detected by the analysis of dependence and withdrawal symptoms presented above. That is, smokers may use cigarettes for a number of reasons, for example, to enhance pleasure, to cope with stress, and to control appetite/body weight. These motivations may not be adequately captured by measures of mood during the experimental test session or by the FTND questionnaire. One might predict that metabolic activity in reward-related structures (eg, striatum) would be associated with pleasure-seeking aspects of smoking, that amygdala activity would correlate with use of cigarettes to reduce anxiety, and that activity of frontal cortical systems (eg, anterior cingulate cortex) would be associated with behavioral regulation and appetite control. Although these expectations were indeed borne out, a strong correlation between thalamus and smoking for calming effects was also observed, and amygdala activity was related not only to smoking for calming effects, but also to the enjoyable sensory aspects of smoking.
Smoking motives related to satisfaction, pleasure, and stimulation
To summarize the correlations detailed below, activity in a number of brain regions correlated with specific smoking motivations related to enjoyment. Most salient in terms of magnitude and consistency were associations between activity of the putamen and smoking for craving satisfaction as well as for pleasurable relaxation and enjoyment of the sensory aspects of cigarette smoke. Somewhat surprisingly, a strong relationship between enjoyment and prefrontal cortical activity was also seen.
Specifically, smoking when experiencing strong craving (Russell questionnaire) was negatively correlated with the between-session difference (sessions 1 and 3 vs session 2) in activity of the putamen (r=−0.56, p=0.04 for the left hemisphere; r=−0.51, p=0.07 for the right hemisphere) and caudate (significant for right hemisphere only; r=−0.61, p=0.03; F(1,11)=3.31, p=0.096 for the interaction with hemisphere). Ratings of smoking to enhance relaxation (RTSQ) were negatively correlated with the between-session difference in putamen activity (r=−0.80, p=0.001 for the left hemisphere; r=−0.62, p=0.02 for the right hemisphere). Scores on the relaxation scale of the Russell questionnaire were negatively correlated with the between-session difference in midbrain activity (r=−0.69, p=0.01 for the left hemisphere; r=−0.64, p=0.02 for the right hemisphere).
Enjoying the taste and smell of smoke (RTSQ) was negatively correlated with the between-session difference in metabolic activity of the putamen (significant for the right hemisphere only; r=−0.69, p=0.009; hemisphere interaction NS) and amygdala (r=−0.57, p=0.04 for the left hemisphere; r=−0.58, p=0.04 for the right hemisphere; F(1,11)=11.74, p=0.006 for the interaction with hemisphere). Liking the taste of smoke (Russell questionnaire) was positively correlated with the mean activity (across all sessions) of the prefrontal cortex (r=+0.83, p=0.0005 for the left hemisphere; r=+0.76, p=0.005 for the right hemisphere) and temporal cortex (significant for left hemisphere only, r=+0.55, p=0.05; hemisphere interaction NS).
Smoking for stimulation (Russell questionnaire) was also negatively correlated with the difference between session in the activity of the midbrain (significant for the left hemisphere only; r=−0.59, p=0.04; F(1,11)=4.33, hemisphere interaction NS), and was positively correlated with between-sessions difference in activity of the anterior cingulate (significant for the left hemisphere only; r=0.59, p=0.03; p=0.06 for interaction with hemisphere).
Smoking to experience a ‘rush’ (RTSQ) was negatively correlated with the between-session difference in activity of the temporal cortex (significant for right hemisphere only, r=+0.62, p=0.02; hemisphere interaction NS).
Smoking motives related to tranquilization
To summarize the correlations listed below, the strongest relationships were observed between smoking for a calming effect and metabolic activity of the thalamus and amygdala.
Specifically, smoking to calm down (RTSQ) was positively correlated with the between-session difference in the activity of the thalamus (r=+0.87, p=0.0002, for the left hemisphere; r=+0.7, p=0.01 for the right hemisphere), and negatively correlated with the between-session difference in activity of the amygdala (significant for left hemisphere only; r=−0.82, p=0.001; F(1,10)=7.37, p=0.02 for the interaction with hemisphere).
Smoking to feel more confident (RTSQ) was positively correlated with the between-session difference in activity of the pons (significant for right hemisphere only; r=+0.66, p=0.01; F(1,11)=13.51, p=0.004 for the interaction with hemisphere), and was positively correlated with the between-session difference in activity of the left hemisphere temporal cortex (r=+0.53, p=0.06) but negatively correlated with the difference in activity of the right hemisphere temporal cortex (r=−0.59, p=0.03).
Using cigarettes as a ‘crutch’ (Russell questionnaire) was positively correlated with the between-session difference in activity of the anterior cingulate region (significant for left hemisphere only; r=0.57, p=0.04; hemisphere interaction NS).
Smoking motives related to self-control
Smoking to control body weight was positively correlated with the mean metabolic activity (across all sessions) of the anterior cingulate region (r=+0.47, p=0.1 for the left hemisphere, r=+0.56, p=0.046 for the right hemisphere). Smoking to enhance concentration was also positively correlated with the mean metabolic activity of the anterior cingulate region (significant for the right hemisphere only; r=+0.57, p=0.04; F(1,11)=4.92, p=0.048 for the interaction with hemisphere).
Correlations between rCMRglc and Task Performance
The between-session difference in errors of omission was positively correlated with the difference in orbitofrontal cortex activity (r=+0.57, p=0.03=for the left hemisphere; r=+0.53, p=0.05 for the right hemisphere). The between-session difference in errors of commission was positively correlated with the difference in midbrain activity (r=+0.55, p=0.04 for the left hemisphere; r=+0.52, p=0.05 for the right hemisphere).
Correlations between rCMRglc and Age, Gender
Age showed strong positive correlations with brain activity (mean across all sessions) in several regions, including pons (r=+0.64, p=0.01 for the left hemisphere; r=+0.79, p=0.0007 for the right hemisphere), midbrain (r=+0.74, p=0.002 for the left hemisphere; r=+0.80, p=0.0006 for the right hemisphere), thalamus (r=+0.64, p=0.01 for the left hemisphere; r=+0.74, p=0.002 for the right hemisphere), ventral striatum (r=+0.62, p=0.02 for the left hemisphere; r=0.45, p=0.1 for the right hemisphere), amygdala (r=+0.79, p=0.0008 for the left hemisphere; r=+0.76, p=0.002 for the right hemisphere), and temporal cortex (significant for right hemisphere only, r=+0.53, p=0.049; hemisphere interaction NS).
Gender showed a strong association with metabolic activity of several brain regions as well as with the change in metabolic activity across sessions. Men showed lower metabolic activity of the anterior cingulate region in sessions 1 and 3 vs session 2 than did women (significant for the left hemisphere only; mean for men=−0.029 (SD=0.034) vs mean for women=+0.01 (SD=0.02); F(1,12)=6.29, p=0.03; hemisphere interaction NS). In addition, men showed less metabolic activity in the ventral striatum in sessions 1 and 3 vs session 2 (significant for right hemisphere only; F(1,12)=5.88, p=0.03; mean for men=−0.05 (SD=0.03) vs mean for women=0.01 (SD=0.046); F(1,12)=3.92, p=0.07 for the interaction with hemisphere). However, men had a higher mean activity of ventral striatum across all sessions relative to women (left hemisphere mean for men=1.03 (SD=0.093) vs mean for women=0.93 (SD=0.091); F(1,12)=4.70, p=0.05; right hemisphere mean for men=1.04, SD=0.056 vs mean for women=0.96 (SD=0.074); F(1,12)=3.90, p=0.07). Men also showed a greater between-session difference (sessions 1 and 3 relative to session 2) in the left hemisphere amygdala activity (mean for men=+0.04 (SD=0.04) vs mean for women=−0.02 (SD=0.04); F(1,12)=8.78, p=0.01; F(1,12)=4.77, p=0.049 for the interaction with hemisphere) and in the left hemisphere colliculi (mean for men=+0.04 (SD=0.055) vs mean for women=−0.04 (SD=0.06); F(1,12)=5.86, p=0.03; F(1,12)=5.96, p=0.03 for the interaction with hemisphere). Men further showed greater average metabolic activity (across all sessions) of the colliculi than women (significant for the left hemisphere only; mean for men=0.9 (SD=0.061) vs mean for women=0.8 (SD=0.067); F(1,12)=10.66, p=0.007; F(1,12)=3.32, p=0.09 for the interaction with hemisphere), and in the temporal cortex (across all sessions): left hemisphere mean for men=0.90 (SD=0.028) vs mean for women=0.88 (SD=0.023); F(1,12)=3.38, p=0.09; right hemisphere mean for men=0.92 (SD=0.027) vs mean for women=0.89 (SD=0.015); F(1,12)=9.81, p=0.009.
Correlations between rCMRglc and rCBF
We computed the correlations between the change in normalized rCMRglc at session 2 (relative to sessions 1 and 3) and the corresponding changes in normalized rCBF. Results showed no consistent correlations between the two measures, with r-values ranging from −0.5 to +0.5.
Potential Confounding Factors
There were no significant differences across sessions in self-reported consumption of alcohol and caffeinated beverages during the previous weeks, or in sleep quality the night before each session. Plasma nicotine levels at the beginning of the session were generally low, and did not differ significantly across sessions (mean of sessions 1 and 3=4.9 ng/ml (SD=8.2) vs 6.1 ng/ml (SD=7.6) at session 2), confirming compliance with instructions to remove skin patches the evening before session 2.
DISCUSSION
The main findings of our study were that (1) craving for cigarettes and nicotine dependence were reduced by a regimen of smoking denicotinized cigarettes in conjunction with wearing nicotine skin patches and (2) a reduction in metabolic activity of the anterior cingulate cortex (right hemisphere) accompanied the denicotinized cigarette+nicotine patch treatment, and this change in activity was greatest for those subjects who adhered to the denicotinized cigarette treatment regimen.
The reduction in dependence accompanying switching to denicotinized cigarettes in combination with nicotine patch treatment was consistent with previous findings reported using similar procedures (Rose et al, 2006). We hypothesized that the dissociation between the act of smoking and receipt of nicotine would promote extinction of the reinforcing properties of cigarettes, thereby reducing craving during periods of abstinence. The reduction in craving was reversible, in that craving recovered to baseline values within 2 weeks after subjects returned to smoking their customary brands of cigarettes (analysis not presented).
The effect of the denicotinized cigarette+nicotine patch treatment on anterior cingulate activity was consistent with other research relating activity in this region to nicotine administration. For example, Ghatan et al (1998) reported that acute i.v. administration of nicotine decreased cerebral blood flow to the anterior cingulate cortex. Ernst also found that activations in the anterior cingulate cortex during a 2-back task were attenuated by nicotine gum administration. The lateralization of the effect to the right hemisphere was similar to that reported by Ernst et al (2001) following acute nicotine administration (which also included only right-handed subjects), and is also consistent with reports that acute nicotine administration preferentially enhances left hemisphere vs right hemisphere arousal as indexed by electroencephalography (EEG) (Gilbert, 1995) or cerebral blood flow (Rose et al, 2003) measures. Thus, nicotine withdrawal (overnight abstinence) would be expected to have opposite effects, leading to a relative activation of right vs left hemisphere. In the present study, withdrawal symptoms were more severe during abstinence following smoking of nicotine vs denicotinized cigarettes, and thus the finding of decreased anterior cingulate activity following treatment with denicotinized cigarettes concurs with results from previous studies. Brody et al (2004), in the only published study we are aware of measuring effects of smoking cessation treatment on regional brain metabolic activity, also reported reductions in anterior cingulate activation accompanying cue-elicited craving after treatment with bupropion. The present study adds to the previous findings by showing that anterior cingulate activity can be influenced by manipulating nicotine dependence while controlling for duration of nicotine abstinence.
Recent studies have suggested that the anterior cingulate region plays a role in attentional selection, conflict, distress, and error monitoring (Luks et al, 2002; Rainville, 2002; Osaka et al, 2004; Ridderinkhof et al, 2004). Thus, this region might be involved in monitoring processes of coping with withdrawal discomfort while remaining engaged in meeting task demands. The intriguing relationships between anterior cingulate activity assessed during experimental sessions and smoking motivations related to weight regulation and concentration as well as with adherence to the denicotinized cigarette treatment are consistent with a broad role of the anterior cingulate cortex in self-control. Impaired self-control mechanisms during smoking cessation would likely disrupt attempts to maintain abstinence.
Several other brain regions, notably the orbitofrontal cortex and ventral striatum, showed associations with subjective reports of craving for cigarettes. These brain regions comprise key structures within the brain reward system, which extends from the dopaminergic neurons in the ventral tegmental area to the ventral striatum (nucleus accumbens), with projections to the orbitofrontal, anterior cingulate, and prefrontal cortices (Balfour et al, 2000; Di Chiara, 2000; Grillner and Svensson, 2000; Mameli-Engvall et al, 2006). It has been hypothesized that nicotine, like other psychostimulants, sensitizes the reward system, engendering craving (Robinson and Berridge, 2003; DiFranza and Wellman, 2005). In studies of cigarette craving, Brody et al (2002, 2004) found that an increase in the metabolic activity of the orbitofrontal cortex was positively correlated with craving for cigarettes.
In contrast to previous studies showing a positive correlation between orbitofrontal cortex activity and craving, our study found a significant negative correlation. Possibly this finding can be accounted for by an important procedural difference, that is we measured brain metabolic activity during performance of an auditory task, whereas the previous studies presented cues designed to elicit craving (eg, Brody et al, 2002; David et al, 2005). If brain activity in these regions reflects responses to rewarding stimulation, then perhaps the level of activity in the absence of craving-associated cues reflected nondrug reward activation, and hence was negatively associated with the reported level of craving. Interestingly, the positive correlation between task-related errors of omission and orbitofrontal cortical activity may reflect difficulty in inhibiting responses to distracting stimuli, in view of the well-documented function of that region in impulse control and decision-making (Bechara et al, 2000; London et al, 2000).
The interpretation of the negative association between craving and regional brain activity as arising from competing nondrug stimuli is supported by the finding that drug withdrawal has been associated with underactivity in the mesolimbic dopamine reward system (Rahman et al, 2004; Kenny and Markou, 2005; Koob and Le Moal, 2005). Insufficient activity in the dopamine reward system activity might plausibly be related to the phasic hyper-responsivness to cues predictive of drug reward (Kalivas and Volkow, 2005). The distinction between tonic state and phasic reactivity to cues should therefore be borne in mind when interpreting brain imaging results. Future studies might further evaluate this hypothesis by assessing regional brain metabolic activity during tasks as well as cue-elicited craving procedures and resting conditions. The sign of the correlation between brain activity and craving would be predicted to change as a result of the cue and task manipulations; a positive correlation between craving and orbitofrontal cortex activation would be predicted when smoking-related cues are presented, whereas a negative correlation would be expected during a cognitive task. A nontask resting condition might produce intermediate and variable results, depending on whether attention is focused on internal withdrawal-associated somatic cues or thoughts of smoking as opposed to nonsmoking related cues.
Our results extend previous work showing the involvement of ventral striatum in nicotine craving and reward in that dorsal striatum (caudate, putamen) also showed significant correlations with reported smoking motivations of craving satisfaction and smoking for pleasurable relaxation. Although many accounts have stressed the role of the ventral striatum, and more specifically nucleus accumbens, in drug craving and reinforcement, several recent studies have provided evidence for dorsal striatum involvement in craving for drugs such as alcohol and cocaine (Heinz et al, 2005; Volkow et al, 2006; Wong et al, 2006).
The correlational analyses relating brain activity to indices of nicotine dependence also showed a strong relationship between increases in thalamic metabolic activity and changes in FTND scores as well as smoking for calming effects. Desire for calming effects, that is to reduce unpleasant affect or arousal, is a motive frequently cited by cigarette smokers in questionnaire studies (Russell et al, 1974; Gilbert et al, 2000; Lujic et al, 2005), and other work suggests that smokers use cigarettes to enhance attentional filtering of unwanted distractions or aversive stimuli. For example, Kassel and co-workers (Kassel and Shiffman, 1997; Kassel and Unrod, 2000) present evidence that the anxiolytic effects of smoking may derive from stimulus gating rather than tranquilization per se. The thalamus plays an important role in sensory gating, and a substantial body of research suggests that nicotine may reverse sensory-gating deficits in individuals with significant deficits, such as patients with schizophrenia or their first-degree relatives (Adler et al, 1992, 1993). Thus, the increase in thalamic activity accompanying deprivation after smoking nicotine-containing vs denicotinized cigarettes may reflect a deficit in sensory gating, and possibly would result in a reduced ability to cope with stressful stimuli, especially in the most highly dependent smokers for whom calming effects were important. Moreover, the thalamus has one of the highest densities of nicotinic receptors in the brain (Clarke, 1990; Tribollet et al, 2004), lending additional credence to the possibility that it plays a pivotal role in nicotine addiction. The role of the thalamus may extend beyond filtering exteroceptive or interoceptive stimuli associated with nicotine withdrawal, and may entail inhibition of responding to stimuli signaling proximal reward and facilitating responding for delayed rewards (Lauwereyns, 2006).
A further association arising from the correlational analyses was that the between-session change in amygdala activity (left hemisphere) was negatively associated with reported smoking for calming effects. The role of the amygdala in modulating anxiety-related behavior is well established (LeDoux, 2003), and some previous brain imaging studies have found that acute nicotine administration decreases amygdala activity (Zubieta et al, 2001; Rose et al, 2003). These results as well as the correlation with smoking motivation observed in the present study support a role for the amygdala in the anxiolytic effects of cigarette smoking. In addition, however, the between-session change in amygdala activity was correlated with reported enjoyment of the taste and smell of cigarette smoke. Although this finding is not altogether surprising in view of the demonstrated role of amygdala in positive reward evaluation as well as in anxiety (Paton et al, 2006), the difference between left and right hemisphere amygdala in terms of the sign of the correlations observed remains unexplained at the present time.
Considering the previous findings together suggests that during short-term cigarette deprivation, dependent smokers may be suffering not only from impaired self-regulation owing to changes in anterior cingulate functioning, excessive sensitivity to cue-elicited craving mediated via dysregulated striatal dopamine reward pathways, and altered affective responses from disruptions in amygdala activity, but also from a deficient thalamic filtering system. This multiplicity of deficits might account for the difficulty smokers report with continual thoughts of smoking that they cannot rid themselves of, and for persistence of craving despite the relative weak primary reinforcing effects of nicotine in relation to other drugs of abuse such as cocaine (Palmatier et al, 2006).
The strong correlations observed between age and metabolic activity of several brain regions may reflect an underlying association with the length of smoking history (and consequently greater dependence), as opposed to an effect of age per se. A nondeprived subject sample (as well as a nonsmoker control group) would shed light on this issue. Similarly, the gender differences noted cannot be ascribed to gender alone as opposed to an interaction of gender with abstinence state or other variables.
In this study, the cerebral blood flow changes accompanying the treatment showed little or no correlation with corresponding changes in regional brain metabolic rate. Although changes in regional cerebral blood flow often reflect metabolic demand, several factors could have accounted for their dissociation. First, blood flow was assessed over a very brief time (1 min), whereas metabolic rate reflected a measure integrated over approximately 30 min, resulting in less variability. Second, subjects performed a task during the assessment of metabolic activity, which could have revealed effects of nicotine deprivation that were not apparent when subjects were resting. Third, the limited sample size and use of change score measures might also have limited the power to detect any correlations. Further studies will be necessary to assess whether cerebral blood flow measurements may be useful in tracking changes in state accompanying manipulations of nicotine dependence.
A major strength of the study design was the use of a within-subject manipulation of nicotine dependence, which may have provided a sensitive tool with which to detect relationships between brain activity and smoking motivation. However, the study also had several limitations that should be acknowledged. The sample size was small, and thus follow-up studies are warranted to replicate the results. Nonetheless, the finding that anterior cingulate activity responded to the manipulation of nicotine dependence is in accord with the Brody et al (2004) study. Moreover, in a recent study using fMRI, we have found that a similar manipulation of nicotine dependence (use of low nicotine content cigarettes in conjunction with NRT) also attenuated blood oxygen level-dependent (BOLD) signal responses to cigarette-related cues in the right hemisphere anterior cingulate cortex (McClernon et al, 2006).
An additional limitation of the study is that the treatment, entailing smoking denicotinized cigarettes in combination with 21 mg/24 h nicotine patches, did not maintain baseline daily nicotine intake (as assessed by saliva cotinine measurements). Thus, not only was the contingency between the act of smoking and nicotine reinforcement disrupted by the treatment, but also the overall level of nicotine exposure was reduced. The effects observed on craving as well as brain activity may have resulted from adaptation to the reduced nicotine levels occurring over 2 weeks, apart from the behavioral extinction resulting from use of denicotinized cigarettes. Future studies might use a higher dose of nicotine skin patches (eg, 42 mg/24 h) to maintain baseline nicotine levels, allowing a more clear dissociation between the effects of removing the contingency between smoking behavior and the delivery of nicotine as opposed to reducing overall nicotine exposure.
Finally, scanning sessions were conducted when subjects were overnight abstinent from smoking, and the absence of a satiation control precludes drawing definitive conclusions about whether changes in subjective and brain responses during session 2 were the result of changes in response to smoking abstinence as opposed to other effects of the treatment or the auditory task. An inclusion of a smoking satiation condition in future studies will be informative.
In summary, the present results add to the growing literature implicating specific brain regions in the modulation of nicotine dependence and cigarette craving. These regions appear to include thalamic gating processes in addition to limbo-cortical and striatal pathways subserving behavioral regulation and reinforcement. Further investigations of these mechanisms, using pharmacologic as well as nonpharmacologic interventions, promise to increase our understanding of the nature of cigarette addiction and may lead to the development of novel treatments for interfering with the dependence process.
References
Adler LE, Hoffer LD, Griffith J, Waldo MC, Freedman R (1992). Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry 32: 607–616.
Adler LE, Hoffer LD, Wiser A, Freedman R (1993). Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 150: 1856–1861.
Balfour DJ, Wright AE, Benwell ME, Birrell CE (2000). The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res 113: 73–83.
Bechara AH, Damasio H, Damasio AR (2000). Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10: 295–307.
Breiter HC, Rosen BR (1999). Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci 877: 523–547.
Brody AL, Mandelkern MA, Lee G, Smith LE, Sadeghi M, Saxena S et al (2004). Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res 130: 269–281.
Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG et al (2002). Brain metabolic changes during cigarette craving. Arch Gen Psychiatry 59: 1162–1172.
Clarke PBS (1990). The central pharmacology of nicotine: electrophysiological approaches. Nicotine Psychopharmacol: Mol, Cell, Behav Aspects. Oxford University Press: New York. pp 158–193.
Cruikshank JM, Neil-Dweyer G, Dorrance DE, Hayes Y, Patel S (1989). Acute effects of smoking on blood pressure and cerebral blood flow. J Hum Hypertens 3: 443–449.
David SP, Munafo MR, Johansen-Berg H, Smith S, Rogers R, Matthews PM et al (2005). Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry 58: 488–494.
DeGrado TR, Hanson MW, Turkington TG, Delong DM, Brezinski DA, Vallee JP et al (1996). Myocardial blood flow estimation for longitudinal studies using 13N-ammonia and PET. J Nucl Cardiol 3: 494–507.
DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE et al (1994). Performance characteristics of a whole-body PET scanner. J Nucl Med 35: 1398–1406.
Di Chiara G (2000). Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol 393: 295–314.
DiFranza JR, Wellman RJ (2005). A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: integrating the clinical and basic science literature. Nicotine Tob Res 7: 9–26.
Domino EF, Minoshima S, Guthrie SK, Ohl L, Ni L, Koeppe RA et al (2000). Effects of nicotine on regional cerebral glucose metabolism in awake resting tobacco smokers. Neuroscience 101: 277–282.
Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta JK et al (2004). Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog Neuropsychopharmacol Biol Psychiatry 28: 319–327.
Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE et al (2001). Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci USA 98: 4728–4733.
Federal Trade Commission (2000). Tar, Nicotine and carbon monoxide of the smoke of 1294 varieties of domestic cigarettes for the year 1998.
Flaum M, O'Leary DS, Cizadlo T, Arndt SV, Hichwa R, Kirchner P et al (1994). Acute effects of cigarette smoking on cerebral blood flow: A PET study. American College of Neuropsychopharmacology, 33rd annual meeting. San Juan, Puerto Rico, December 12–16, 1994. Scientific Abstracts, p. 148.
Fox PT, Mintun MA, Raichel ME, Miezin FM, Allman JM, Van Essen DC (1986). Mapping the human visual cortex with positron emission tomography. Nature 323: 806–809.
Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K et al (1998). Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology (Berlin) 136: 179–189.
Gilbert DG (1995). Smoking: Individual Differences, Psychopathology, and Emotion. Taylor & Francis: London.
Gilbert DG, Sharpe JP, Ramanaih NV, Detwiler FRJ, Anderson AE (2000). Development of a situation x trait adaptive response (STAR) model-based smoking motivation questionnaire. Pers Indiv Differ 29: 65–84.
Grillner P, Svensson TH (2000). Nicotine-induced excitation of midbrain dopamine neurons in vitro involves ionotropic glutamate receptor activation. Synapse 38: 1–9.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991). The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict 86: 1119–1127.
Heinz AT, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y et al (2005). Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry 162: 1515–1520.
Hyman SE, Malenka RC, Nestler EJ (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598.
Kalivas PW, Volkow ND (2005). The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162: 1403–1413.
Kassel JD, Shiffman S (1997). Attentional mediation of cigarette smoking's effect on anxiety. Health Psychol 16: 359–368.
Kassel JD, Unrod M (2000). Smoking, anxiety, and attention: support for the role of nicotine in attentionally mediated anxiolysis. J Abnorm Psychol 109: 161–166.
Kenny PJ, Markou A (2005). Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci 25: 6208–6212.
Koob GF, Le Moal M (2005). Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8: 1442–1444.
Lauwereyns J (2006). Voluntary control of unavoidable action. Trends Cogn Sci 10: 47–49.
LeDoux J (2003). The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727–738.
London ED, Ernst M, Grant S, Bonson K, Weinstein A (2000). Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex 10: 334–342.
Lujic C, Reuter M, Netter P (2005). Psychobiological theories of smoking and smoking motivation. Eur Psychol 10: 1–24.
Luks TL, Simpson GV, Feiwell RJ, Miller WL (2002). Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. Neuroimage 17: 792–802.
Madden DJ, Langley LK, Denny LL, Turkington TG, Provenzale JM, Hawk TC et al (2002). Adult age differences in visual word identification: functional neuroanatomy by positron emission tomography. Brain Cogn 49: 297–321.
Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP et al (2006). Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50: 911–921.
Mathew RJ, Wilson WH (1995). Acute pharmacological effects of tobacco smoking and nicotine on cerebral circulation. In: Domino EF (ed). Brain Imaging of Nicotine and Tobacco Smoking. NPP Books: Ann Arbor. pp 109–121.
Mayes SD, Calhoun SL (1999). Discriminative validity of the Gordon Diagnostic System (GDS). ADHD Rep 7: 11–14.
McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE (2006). Extinction-based smoking cessation treatment attenuates event-related brain responses to smoking cues. Poster presented at the meeting of the College on Problems of Drug Dependence, Orlando, Florida.
Nagata K, Shinohara T, Kanno I, Hatazawa J, Domino EF (1995). Effects of tobacco cigarette smoking on cerebral blood flow in normal adults. In: Domino EF (ed). Brain Imaging of Nicotine and Tobacco Smoking. NPP Books: Ann Arbor, MI. pp 95–107.
Osaka N, Osaka M, Morishita M, Kondo H, Fukuyama H (2004). A word expressing affective pain activates the anterior cingulate cortex in the human brain: an fMRI study. Behav Brain Res 153: 123–127.
Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donney EC et al (2006). Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berlin) 184: 391–400.
Paton JJ, Belova MA, Morrison SE, Salzman CD (2006). The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439: 865–870.
Pelizzari CA, Chen GT, Spelbring DR, Weichselbaum RR, Chen CT (1989). Accurate three-dimensional registration of CT, PET and/or MR images of the brain. J Comput Assist Tomogr 13: 20–26.
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE (1979). Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 6: 371–388.
Rahman S, Zhang J, Engleman EA, Corrigall W (2004). Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: a microdialysis study. Neuroscience 129: 415–424.
Rainville P (2002). Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol 12: 195–204.
Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS (2004). Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 56: 129–140.
Robinson TE, Berridge KC (2003). Addiction. Annu Rev Psychol 54: 25–53.
Rose JE, Behm FM, Westman EC, Kukovich P (2006). Pre-cessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res 8: 89–101.
Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC et al (2003). PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiatry 160: 323–333.
Russell MAH, Peto J, Patel UA (1974). The classification of smoking by factorial structure of motives with discussion. J R Stat Soc 137: 313–346.
Shiffman SM, Jarvik ME (1976). Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology 50: 35–39.
Skinhoj E, Olesen J, Paulson OB (1973). Influence of smoking and nicotine on cerebral blood flow and metabolic rate of oxygen in man. J Appl Physiol 35: 820–822.
Solti F, Peter A, Olah I, Iskum M, Rev J, Hermann R et al (1963). Effect of nicotine on cerebral blood flow and cerebral venous pressure. Cor Vasa 5: 197–202.
Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF et al (2003). Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology 28: 765–772.
Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG et al (1998). Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 155: 1009–1015.
Tribollet E, Bertrand D, Marguerat A, Raggenbass M (2004). Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience 124: 405–420.
Turkington TG, Hoffman JM, Jaszczak RJ, MacFall JR, Harris CC, Kilts CD et al (1995). Accuracy of surface fit registration for PET and MR brain images using full and incomplete brain surfaces. J Comput Assist Tomogr 19: 117–124.
U.S.D.H.H.S (1988). The Health Consequences of Smoking: Nicotine Addiction. Office on Smoking and Health: Rockville, MD.
Van Selst M, Jolicouer P (1994). A solution to the effect of sample size on outlier elimination. Quart J Exp Psychol Hum Exp Psychol 47: 631–650.
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR et al (2006). Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26: 6583–6588.
Wennmalm A (1982). Effect of cigarette-smoking on basal and carbon dioxide stimulated cerebral blood flow in man. Clin Physiol 2: 529–535.
Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A et al (2006). Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 12: 2716–2727.
Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL et al (2005). Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry 58: 143–150.
Zubieta J, Lombardi U, Minoshima S, Guthrie S, Ni L, Ohl LE et al (2001). Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol Psychiatry 49: 906–913.
Acknowledgements
This research was supported by the External Research Program of Philip Morris USA, Inc. and Philip Morris International Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rose, J., Behm, F., Salley, A. et al. Regional Brain Activity Correlates of Nicotine Dependence. Neuropsychopharmacol 32, 2441–2452 (2007). https://doi.org/10.1038/sj.npp.1301379
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301379
Keywords
This article is cited by
-
The Periaqueductal Gray and Its Extended Participation in Drug Addiction Phenomena
Neuroscience Bulletin (2021)
-
Altered spontaneous activity of posterior cingulate cortex and superior temporal gyrus are associated with a smoking cessation treatment outcome using varenicline revealed by regional homogeneity
Brain Imaging and Behavior (2017)
-
Altered resting state functional connectivity of anterior insula in young smokers
Brain Imaging and Behavior (2017)
-
Betel quid dependence is associated with functional connectivity changes of the anterior cingulate cortex: a resting-state fMRI study
Journal of Translational Medicine (2016)
-
Increased functional connectivity in the resting-state basal ganglia network after acute heroin substitution
Translational Psychiatry (2015)