Abstract
Preclinically, the combination of an SSRI and 5-HT autoreceptor antagonist has been shown to reduce the time to onset of anxiolytic activity compared to an SSRI alone. In accordance with this, clinical data suggest the coadministration of an SSRI and (±) pindolol can decrease the time to onset of anxiolytic/antidepressant activity. Thus, the dual-acting novel SSRI and 5-HT1A/B receptor antagonist, SB-649915-B, has been assessed in acute and chronic preclinical models of anxiolysis. SB-649915-B (0.1–1.0 mg/kg, i.p.) significantly reduced ultrasonic vocalization in male rat pups separated from their mothers (ED50 of 0.17 mg/kg). In the marmoset human threat test SB-649915-B (3.0 and 10 mg/kg, s.c.) significantly reduced the number of postures with no effect on locomotion. In the rat high light social interaction (SI), SB-649915-B (1.0–7.5 mg/kg, t.i.d.) and paroxetine (3.0 mg/kg, once daily) were orally administered for 4, 7, and 21 days. Ex vivo inhibition of [3H]5-HT uptake was also measured following SI. SB-649915-B and paroxetine had no effect on SI after 4 days. In contrast to paroxetine, SB-649915-B (1.0 and 3.0 mg/kg, p.o., t.i.d.) significantly (p<0.05) increased SI time with no effect on locomotion, indicative of an anxiolytic-like profile on day 7. Anxiolysis was maintained after chronic (21 days) administration by which time paroxetine also increased SI significantly. 5-HT uptake was inhibited by SB-649915-B at all time points to a similar magnitude as that seen with paroxetine. In conclusion, SB-649915-B is acutely anxiolytic and reduces the latency to onset of anxiolytic behavior compared to paroxetine in the SI model.
Similar content being viewed by others
INTRODUCTION
Selective serotonin (5-hydroxytryptamine, 5-HT) reuptake inhibitors (SSRIs) are widely used, clinically efficacious antidepressant and anxiolytic agents. However, one of their major drawbacks, as with all antidepressants, is the time to onset of clinical effect, which can typically take 3–4 weeks. It has been hypothesized that this delayed efficacy of SSRIs may be alleviated by the concurrent blockade of 5-HT1A autoreceptors and serotonin reuptake inhibition. Artigas and colleagues (1994) reported that coadministration of paroxetine and the 5-HT1A receptor partial agonist and β-adrenergic receptor antagonist, (±) pindolol decreased the time to onset of antidepressant action compared to the SSRI alone from 2–3 weeks to 3–7 days. These findings have been confirmed by numerous groups (Blier and Bergeron, 1995; Zanardi et al, 1997; Bordet et al, 1998; McAskill et al, 1998; Maes et al, 1999) and in a meta-analysis by Ballesteros and Callado (2004), but have been refuted by others (Berman et al, 1997, 1999). It is likely that the recent controversy surrounding the optimal dose of pindolol used in the augmentation of SSRI (Segrave and Nathan, 2005; Rabiner et al, 2001) may explain these inconsistencies.
The delay in clinical efficacy with SSRIs is, in part, thought to be due to their inability to acutely increase extracelluar 5-HT levels. Indeed, this is evident in rodents in which acute SSRI treatment decreases serotonergic cell firing via the activation of somatodendritic 5-HT1A autoreceptors in the dorsal raphe (Blier et al, 1987; Gartside et al, 1995). It is not until the desensitization of these receptors has occurred that a sustained elevation in extracellular 5-HT levels is seen (Hensler et al, 1991; Le Poul et al, 1995; Mongeau et al, 1997). Furthermore, preclinical neurochemical studies have shown that blockade of the inhibitory 5-HT1A autoreceptors with (±) pindolol induces an acute, rapid elevation of extracellular forebrain 5-HT that is not seen with SSRIs alone (Artigas, 1993; Hjorth, 1996; Dawson and Nguyen, 2000). Data with the highly selective 5-HT1A receptor antagonist WAY100635 (Forster et al, 1995; Fletcher et al, 1996) combined with SSRIs provide further evidence to support the role of 5-HT1A receptors in this response (Gartside et al, 1995; Dawson and Nguyen, 1998).
In addition to the 5-HT1A autoreceptor, 5-HT1B/D autoreceptors also play a part in regulating 5-HT release from the nerve terminal (Hoyer and Middlemiss, 1989) and 5-HT synthesis (Hjorth et al, 1995). There is also evidence that 5-HT1B receptor desensitization is seen following chronic SSRI treatment indicating that these receptors may play a part in mediating the effects seen with SSRIs (Blier and Bouchard, 1994; Newman et al, 2004; Shalom et al, 2004). Preclinical microdialysis studies have demonstrated that the selective 5-HT1B receptor antagonist SB-224289 augments the 5-HT increase seen with paroxetine, and that WAY100635 further potentiates this effect (Roberts et al, 1999). Another 5-HT1B/D receptor antagonist, GR127935, also augments an SSRI effect in microdialysis (Rollema et al, 1996; Gobert et al, 1997) and potentiates the effects of an SSRI/5-HT1A receptor antagonist combination (Sharp et al, 1997; Dawson and Nguyen, 2000). Therefore, 5-HT1A/B/D receptor antagonism may play a beneficial role in the augmentation of the effects of SSRIs.
Anxiety tests such as the marmoset human threat test and rat pup ultrasonic vocalization test have been shown to identify clinically efficacious anxiolytic and antidepressant agents, for example, benzodiazepines and SSRIs (Gardner, 1985; Olivier et al, 1998; Costall et al, 1988b). However, although these studies may, at least in part, predict the clinical efficacy of this class of compounds, they do not model the temporal effects of SSRIs seen in the clinic. Lightowler et al (1994) demonstrated that in the high light social interaction (SI) test, a rodent test of anxiety-related behavior (File and Hyde, 1978, 1979; File, 1980), chronic, but not acute, administration of paroxetine significantly increased time spent in active SI (indicative of an anxiolytic profile). These data demonstrated that the high light SI test may be a useful model to evaluate the temporal nature of SSRI-induced anxiolysis. Furthermore, Duxon et al (2000) reported that coadministration of paroxetine and WAY100635 reduced the latency to anxiolysis from 21 days with paroxetine alone to 7 days with the combination. Hence, these findings suggest that the rat high light SI test may be used to model the rapid onset of action seen in the clinic with the combination of an SSRI and (±) pindolol.
We report here the behavioral assessment of SB-649915-B, a novel SSRI and 5-HT1A/B autoreceptor antagonist (Atkinson et al, 2005) that has high affinity for recombinant human and rat native tissue 5-HT1A and 5-HT1B receptors (Scott et al, 2006). [3H]citalopram binding and [3H]5-HT uptake studies have also shown that SB-649915-B has high affinity for human recombinant and rat native SERT (Scott et al, 2006). SB-649915-B also has a good selectivity profile against other 5-HT, dopamine, and adrenergic (α1B and β2) receptors (Atkinson et al, 2005; Scott et al, 2006). In vivo SB-649915-B shows antagonism at 5-HT1A and 5-HT1B receptors as determined by the dose-dependent inhibition of 8-OH-DPAT-induced hyperlocomotion and SKF-99101-H-induced increase in seizure threshold (Hughes et al, 2007) respectively. Ex vivo [3H]5-HT uptake studies have also determined the functional activity of SB-649915-B at the serotonin transporter following oral dosing in rodents (Hughes et al, 2007). The studies described herein determine the effects of SB-649915-B after acute administration in the rat pup ultrasonic vocalization and marmoset human threat tests, and evaluate the temporal nature of these effects in the high light SI model.
METHODS
Animals
All in vivo studies conformed to GlaxoSmithKline ethical standards and were conducted in accordance with either the United Kingdom Animals (Scientific Procedures) Act, 1986 or Italian law (art. 7, Legislative Decree no. 116, 27 January 1992), which acknowledges the European Directive (86/609/EEC).
Male Sprague–Dawley rat pups (Charles River, Italy) used in the vocalization study were housed under standard laboratory conditions on a 12 h light/dark cycle with lights on at 0500 GMT and food and water available ad libitum.
Human threat test studies utilized laboratory-bred male (vasectomized) and female common marmosets over 2 years of age, weighing 300–500 g. The animals were caged in couples, in a housing room maintained at 25±1°C, 60% humidity, and a 12 h light/dark cycle (lights on at 0500 GMT, with 30 min-simulated dawn and twilight). Water was available ad libitum and food was administered twice daily (0700 and 1400 h).
In the SI studies, male Sprague–Dawley rats (Charles River, UK, 265–384 g on the day of testing) were group-housed until 5 days before testing, after which they were singly housed to increase their drive for SI. Animals were housed under standard laboratory conditions, had free access to food and water, and were maintained under a 12 h light/dark cycle with lights on at 0600 GMT.
Ultrasonic Vocalizations in Rat Pups
Rat pups were housed with their mothers and littermates under standard laboratory conditions. At the age of 9–12 days, pups were screened over a 1 min session for their ability to emit ultrasonic vocalization (42 kHz) after removal from their home cage. During the test period, animals were maintained at 22±1°C and their vocalizations were recorded in a soundproof box using a microphone. The signal was filtered and transformed into digital block pulse and was processed by a dedicated software (Ultravox system, Noldus, The Netherlands). Only subjects with duration of vocalization longer than 20 s over the 1 min training session were included in the test session. Immediately following the training session, animals were returned to their home cages and randomly assigned to treatment groups (n=6–8). The test and training sessions occurred on the same day and vocalizations were recorded over a 5 min test period.
Human Threat Test in Marmosets
Both marmosets in each housing pair were involved in the test, which was carried out with the animals situated in their home cage. The behavioral response (number of postures, see Costall et al, 1988a) to a human observer standing in close proximity to the home cage was recorded. Only animals that in the week before the test met the criterion of >10 postures over a 2 min session were included in the experimental design. Drug treatments were assigned according to a blind crossover design. The number of postures and jumps from the back of the cage to the cage front was measured during the 2 min test to assess potential anxiolytic-like activity and exclude sedation or locomotor stimulation (n=4). After a washout period of at least 3 days, treatments were reassigned and the study was completed when all animals had received all treatments.
Social Interaction Test
Rats were allocated to a test pair on the basis of weight (max±21 g) and both received the same treatment (n=10 or 12 individuals). SI was determined in a white perspex test arena (54 (width) × 36 (depth) × 29 (height) cm), with a solid white floor divided into 24 squares (9 × 9 cm). A transparent perspex front allowed behavior to be monitored using a video camera positioned in front of the arena and linked to a video recorder and monitor in an adjoining room. Lighting was provided using normal lighting levels augmented by a lamp with a 60 W bulb placed directly above the arena. All behavior was observed between 0900 and 1700 in 5 pair blocks with each treatment represented once within each block. Behavior was monitored from videotapes at a later date by an observer blind to treatment. Total time spent in interaction and amount of locomotor activity for each animal was scored over a 15 min test period. Duration of SI (interaction time) was defined as seconds spent sniffing, grooming, boxing, and climbing over and under partner. Locomotion (zone transitions) was defined as number of whole squares transversed on the base of the arena.
Ex Vivo Inhibition of [3H]5-HT Uptake
Immediately after SI testing (2 h 15 min post-dose) some animals (n=3–4 per group) were sacrificed and tissue removed for ex vivo analysis (Thomas et al, 1987). Cortical tissue from each test animal was resuspended in 30 volumes (w/v) of ice-cold tissue buffer (50 mM TRIS Pre-Set, pH 7.7, 10 mM MgCl2) and was homogenized using an Ultra-Turrux. Each tissue sample was then used to generate both total binding and nonspecific binding values in triplicate against [3H]5-HT. Nonspecific binding was determined using 1 μM paroxetine. Samples were incubated at 37 °C for 45 min before the assay was terminated by rapid filtration through GF/B filters presoaked in 0.3% PEI. This was followed by 5 × 1 ml washes with ice-cold harvest buffer (50 mM TRIS Pre-Set, pH 7.7, 10 mM MgCl2). Filters were left to dry before scintillation fluid was added and bound radioactivity determined by scintillation spectrometry.
Drugs and Dosing
SB-649915-B (6-[(1-{2-[(2-methyl-5-quinolinyl)oxy]ethyl}-4-piperidinyl)methyl]-2H-1,4-benzoxazin-3(4H)-1) synthesized by the Department of Medicinal Chemistry, GlaxoSmithKline was administered as mg/kg free base (except to rat pups where doses were expressed as mg/kg salt). Paroxetine (GlaxoSmithKline) and fluoxetine (Medicinal Chemistry, GlaxoSmithKline) were administered as mg/kg salt. All compounds were administered in 1% methylcellulose (Sigma-Aldrich, Poole, UK). Drugs and reagents for ex vivo binding were purchased from Sigma-Aldrich (Poole, UK), Research Biochemicals International (Poole, UK), and Tocris Cookson Ltd (Bristol, UK). [3H]5-HT was supplied by Amersham International (Little Chalfont, UK).
In the vocalization tests pups were treated intraperitoneally (i.p.) at 10 ml/kg with 1% methylcellulose, SB-649915-B (0.1, 0.3, and 1.0 mg/kg) or fluoxetine (10 mg/kg) 30 min before testing. Six hours before testing in the human threat test marmosets were treated subcutaneously (s.c.) at 1 ml/kg with 1% methylcellulose or SB-649915-B (1.0, 3.0, and 10 mg/kg).
In the SI studies 1% methylcellulose or SB-649915-B (1.0, 3.0, or 7.5 mg/kg) was administered by oral gavage (p.o.), at 2 ml/kg, three times daily (t.i.d.; 0800, 1400, and 2000). Animals were dosed using this regiment for 4, 7, or 21 days in three separate experiments (each time point was investigated in an individual experiment). On the final day of treatment (day 4, 7, or 21 of dosing), animals were dosed once 2 h before testing. Paroxetine was administered p.o. once daily (u.i.d.) at the 0800 dosing point and 1 h before testing. On the two subsequent daily dosing occasions these animals received vehicle only. This dosing regimen was based on pharmacokinetic data generated in-house to model the systemic exposure levels in a 24 h period with SB-649915-B (data not shown).
Data Analysis and Statistics
Rat pup ultrasonic vocalization data are expressed as a mean duration of vocalization (±standard error of the mean (SEM)). Data were subjected to a paired t-test, comparing each compound dose with the related vehicle treatment (GB-STAT statistical software, Dynamic Microsystems). ED50 values and 95% confidential limits were calculated using a linear fit procedure using RS1 software (version 4.21, BBN Software Product Corporation) (Draper and Smith, 1966).
Human threat test data are expressed as mean number of postures or jumps±SEM. Data were subjected to a one-way ANOVA, followed by a post hoc (Dunnett's) test (GB-STAT statistical software, Dynamic Microsystems).
SI data were captured as interaction time (seconds) and locomotor activity (number of zone transitions) and presented as mean±SEM for each treatment group. The effects of SB-649915-B and paroxetine vs vehicle control animals were assessed by one-way ANOVA followed by a post hoc t-test (Duncan's) when appropriate (Statistica, SatSoft Inc., OK, USA).
[3H]5-HT uptake was calculated from the specific d.p.m. per treatment group and presented normalized to vehicle±SEM. The effects of SB-649915-B and paroxetine were compared to vehicle controls by one-way ANOVA followed by Duncan's t-test when appropriate (Statistica, SatSoft Inc., OK, USA).
RESULTS
SB-649915-B in the Rat Pup Vocalization Model
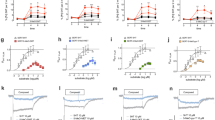
SB-649915-B significantly decreased duration of vocalization in rat pups compared to vehicle at 0.1 (p<0.05), 0.3, and 1.0 mg/kg (p<0.01) with an estimated ED50 of 0.17 mg/kg (95% confidence limits, 0.09–0.26 mg/kg), consistent with an anxiolytic-like activity (Figure 1). The positive control fluoxetine (10 mg/kg) also significantly (p<0.01) reduced duration of vocalization.
Effects of SB-649915-B on ultrasonic vocalizations in rat pups. SB-649915-B (0.1, 0.3, and 1.0 mg/kg) was administered i.p. 30 min before test. Data are expressed as mean duration of vocalization(s)±SEM (n=6–8 per group). SB-649915-B (0.1, 0.3, and 1.0 mg/kg, i.p.) and fluoxetine (10 mg/kg, i.p.) significantly reduced vocalization compared to vehicle. *p<0.05, **p<0.01 from vehicle-treated animals (ANOVA followed by Dunnett's test).
SB-649915-B in the Marmoset Human Threat Test
Acute administration of SB-649915-B significantly (p<0.01). reduced the number of postures compared to vehicle control by 45% and 47% at 3.0 and 10 mg/kg s.c. respectively (F3,12=10.2, p=0.001, Figure 2a). SB-649915 did not affect the number of jumps, suggesting the reduction in postures was not confounded by sedative effects over this dose range (F3,12=18.7, p=0.23; Figure 2b).
Effects of SB-649915-B in the human threat test in marmosets. SB-649915-B (1.0, 3.0, and 10 mg/kg) was administered s.c. 60 min before test. Data are expressed as mean number of postures and jumps±SEM (n=4 per group). SB-649915-B significantly reduced postures with no effect on the number of jumps. **p<0.01 compared to vehicle-treated animals (Dunnett's test).
SB-649915-B on Rat Social Interaction Behavior
(i) Subchronic (4 days) SB-649915-B on rat social interaction behavior
Administration of SB-649915-B (1.0–7.5 mg/kg, p.o., t.i.d.) or paroxetine (3.0 mg/kg, p.o., u.i.d.), for 4 days did not influence either time spent in SI or locomotor activity (F4,55=1.4, p=0.88 interaction; F4,55=1.9, p=0.13 locomotion; Figure 3a) in any of the treatment groups. In a subset of behavioral animals, paroxetine (3.0 mg/kg, p.o., u.i.d.) and SB-649915-B (3.0 and 7.5 mg/kg, p.o., t.i.d.) significantly (p<0.05 and p<0.001 respectively) reduced [3H]5-HT uptake after 4 days administration (F4,10=18.7, p=0.001, 4 days; Figure 3a).
The effect of subchronic (4 and 7 days) and chronic (21 days) SB-649915-B (1.0–7.5 mg/kg, p.o., t.i.d.) or paroxetine (3.0 mg/kg, p.o., u.i.d.) on rat behavior in the high light SI test and SERT occupancy as determined by inhibition of [3H]5-HT uptake. Data are presented as mean interaction time(s)±SEM, mean locomotion (zone transitions)±SEM (n=10–12), and mean % occupancy±SEM (n=3–4). *, **, and *** denote significant change (p<0.05, p<0.01, or p<0.001, respectively) from vehicle-treated animals (Duncan's t-test following ANOVA SI; Dunnett's test following ANOVA SERT occupancy). A significant increase in interaction time was observed with SB-649915-B on day 7 (1.0 and 3.0 mg/kg) and 21 (1.0, 3.0, and 7.5 mg/kg) and paroxetine on day 21 with no concurrent effect on locomotion (b and c). SB-649915-B significantly occupied SERT compared to vehicle. *p<0.05, **p<0.01 from vehicle-treated controls.
(ii) Subchronic (7 days) SB-649915-B on rat social interaction behavior
Administration of SB-649915-B (1.0–7.5 mg/kg, p.o. t.i.d.) or paroxetine (3.0 mg/kg, p.o., u.i.d.) for 7 days was assessed compared to vehicle-treated controls. Paroxetine (3.0 mg/kg, p.o., u.i.d., 7 days) did not affect interaction time or locomotor activity (Figure 3b). SB-649915-B (1.0 and 3.0 mg/kg, p.o., t.i.d., 7 days) significantly (p<0.01) increased interaction time (F4,55=6.4, p=0.0003), but did not influence locomotor activity (F4,55=0.7, p=0.59) (Figure 3b). In contrast, the top dose of SB-649915-B tested (7.5 mg/kg, p.o., t.i.d., 7 days) failed to show effect either on interaction time or locomotor activity. [3H]5-HT uptake was assessed in a group of animals that had undergone behavioral testing and was significantly reduced (F4,15=40.7, p=0.0001, 7 days; Figure 3b) following 7 days administration of paroxetine (p<0.001) and SB-649915-B 1.0 (p<0.05), 3.0 (p<0.001), and 7.5 mg/kg (p<0.001).
(iii) Chronic (21 days) SB-649915-B on rat social interaction behavior
Administration of SB-649915-B (1.0–7.5 mg/kg, p.o., t.i.d.) for 21 days significantly (p<0.01) increased interaction time with no significant concurrent increase in locomotor activity compared to vehicle-treated controls. Paroxetine (3 mg/kg, p.o., u.i.d.)-treated animals also significantly increased interaction time with no effect on locomotor activity (F4,53=5.5, p=0.003 interaction; F4,53=2.4, p=0.06 locomotion; Figure 3c). Following 21 days dosing, [3H]5-HT uptake was significantly reduced (F4,15=7.3, p=0.002, 21 days; Figure 3c) in a subset of the behavioral animals following paroxetine (p<0.001). SB-649915-B also significantly (p<0.001) inhibited [3H]5-HT uptake in a dose-dependent manner at 3.0 and 7.5 mg/kg. The effect at 1.0 mg/kg was also not significant; however the magnitude of the effect was as that at 7 days.
DISCUSSION
SSRIs are well established in the treatment of anxiety disorders (such as panic disorder, obsessive compulsive disorder, generalized anxiety disorder, and posttraumatic stress disorder) (for review see Nutt, 2005) and depression. However, it is widely established that it can take several weeks for clinical efficacy to be seen. It has been suggested that this delayed onset of clinical effect may be due, at least in part, to the need for desensitization of somatodendritic 5-HT1A autoreceptors in the dorsal raphe (Le Poul et al, 1995). Hence, it has been hypothesized that concomitant therapy with an SSRI increasing 5-HT in the forebrain and a 5-HT1A receptor antagonist preventing feedback via blockade of the autoreceptors may result in a reduction of the time to onset of efficacy in the clinic. In support of this hypothesis, clinical data have indicated that the coadministration of an SSRI with the mixed β-adrenergic and 5-HT1A receptor antagonist (±) pindolol can hasten the commencement of antidepressant efficacy from several weeks to between 3 and 7 days (Artigas et al, 1994; Blier and Bergeron, 1995), although there has been evidence to the contrary in depression (Berman et al, 1997, 1999) and anxiety (Stein et al, 2001). There are also a substantial number of preclinical rodent neurochemistry studies that support the proposal that the increase in forebrain 5-HT seen with SSRIs after chronic administration can been seen acutely with concurrent blockade of the 5-HT1A autoreceptors (Dawson and Nguyen, 1998; Gartside et al, 1995). In addition, 5-HT1B/D antagonists augment an SSRI-induced increase in 5-HT (Dawson and Nguyen, 2000) and further potentiate the increase in 5-HT following WAY100635 and fluoxetine (Gobert et al, 2000).
With this in mind, a novel 5-HT1A/B autoreceptor antagonist and 5-HT reuptake inhibitor SB-649915-B has been developed (Atkinson et al, 2005). SB-649915-B occupies and fully blocks the serotonin transporter and 5-HT1A/B receptors whilst inducing an increase in extracellular 5-HT in the forebrains of guinea pigs and rats following acute administration (Hughes et al, 2007). Here, we evaluate the effects of SB-649915-B following acute and chronic administration in preclinical tests of anxiety-like behavior.
SSRIs have been shown to have acute behavioral effects in a number of preclinical assays of anxiety (for review, see Borsini et al, 2002). When rat pups are separated from their mothers they emit ultrasonic vocalizations which can be attenuated by acutely administered anxiolytic agents such as benzodiazepines and SSRIs (Gardner, 1985; Olivier et al, 1998). In the rat pup, vocalization was reduced in a dose-dependent manner by SB-649915-B, and at the top dose (1.0 mg/kg) this was to an equivalent degree as the SSRI, fluoxetine (10 mg/kg). Based on these acute findings in the rodent model, the studies were extended to a primate species, the common marmoset, in the human threat test. When confronted by a human in close proximity to their home cage, marmosets show a series of behavioral postures that are considered to be related to their level of anxiety (Costall et al, 1988a). These postures can be reduced by administration of anxiolytic agents at doses that have no sedative effects as measured by a decrease in the number of jumps (Costall et al, 1988a, 1988b). SB-649915-B significantly reduced the number of postures at 3.0 and 10 mg/kg with no effect on the number of jumps, demonstrating a specific anxiolytic effect in a nonhuman primate.
The mechanism of action in the pup vocalization model is thought to be 5-HT related as only compounds with serotonergic activity (eg SSRIs, clomipramine, and impiramine) show efficacy in this assay (for review, see Borsini et al, 2002). However, a small or no increase in 5-HT has been measured using microdialysis following acute SSRI administration (Sharp et al, 1997; Roberts et al, 1999; Dawson and Nguyen, 2000) questioning whether the acute anxiolytic effects are due to changes in 5-HT levels or whether other neurotransmitter systems play a role. It could be postulated that an overflow technique such as microdialysis simply lacks the sensitivity to measure small changes in 5-HT, and increases in synaptic 5-HT are sufficient to mediate the observed acute anxiolytic effect. Alternatively, SSRIs and 5-HT1A/B receptor antagonists have been shown to modulate several other neurotransmitter systems including glutamate (Marcoli et al, 1999; Schechter et al, 2002), acetylcholine (Consolo et al, 1996; Nakai et al, 1998; Koyama et al, 1999), dopamine (Boulenguez et al, 1996; Nomikos et al, 1996; Iyer and Bradberry, 1996), and noradrenaline (Hajos-Korcsok et al, 1999) which may contribute to or mediate the SB-649915-B-induced effect. However, the studies to address the effects on systems other than the 5-HT and monoaminergic (Hughes et al, 2007), have, as yet, not been performed.
The data from the rat pup vocalization and marmoset human threat test studies suggest that SB-649915-B has an anxiolytic-like profile in a rodent and primate species following acute dosing. These acute data are encouraging and indicate that the presence of 5-HT1A/B antagonism does not adversely affect the SSRI response in these anxiety models. However, as SSRIs may take several weeks to produce therapeutically beneficial effects, there is a need to introduce novel agents that are equally efficacious against the symptoms of depression and anxiety, as established in acute models, yet exhibit a faster onset of action. Several preclinical models (eg elevated plus maze, social defeat model, reversal of cholecystokinin, or CRH-induced anxiety in the SI) are able to model the delay in onset to activity that is evident in the clinic (Griebel et al, 1994, 1995; Berton et al, 1999; To and Bagdy, 1999; To et al, 1999). The studies by Lightowler et al (1994) and Duxon et al (2000) demonstrated that the rat high light SI could be used to predict the onset of anxiolytic activity of paroxetine after chronic (21 days) dosing. Further, Duxon et al (2000) demonstrated that the concomitant administration of paroxetine with WAY100635 reduced the latency to onset of anxiolytic behavior from 21 to 7 days. In the light of this data we have chosen to use the SI test of anxiety-like behavior to determine the rate of anxiolytic onset of the combined SSRI and 5-HT1A/B receptor antagonist SB-649915-B.
Consistent with previous findings chronic (21 days), but not subchronic (4 and 7 days) treatment with the SSRI paroxetine increased SI with no concurrent effect on locomotor activity, indicative of a delayed onset of anxiolytic effect (Lightowler et al, 1994; Duxon et al, 2000). In contrast to paroxetine, SB-649915-B dosed for 7 days significantly increased SI with no effect on locomotor activity and this effect was still evident after 21 days dosing. SI was also monitored after 4 days administration, but there was no significant effect of SB-649915-B at this earlier time point. Ex vivo uptake in cortical tissue isolated from behavioral animals showed a decrease in [3H]5-HT uptake at 4, 7, and 21 days dosing following paroxetine and SB-649915-B. The lack of behavioral efficacy of paroxetine until day 21, therefore, is not owing to a lack of SERT activity. Because SB-649915-B also produced a robust dose-related occupancy of SERT at all time points, the apparent hastened onset of efficacy is therefore likely to be due to the combination of an SSRI and 5-HT1A/B receptor antagonism in this molecule. The adaptive changes required for SB-649915-B to show anxiolytic behavior potentially occur between days 4 and 7 in this assay, whereas the paroxetine effects have been postulated to occur between days 14 and 21 of administration (Lightowler et al, 1994). There are also a substantial number of preclinical rodent neurochemistry studies that support the proposal that the increase in forebrain 5-HT seen with SSRIs alone after chronic administration can be seen acutely with concurrent blockade of the 5-HT1A autoreceptors using (±) pindolol (Dreshfield et al, 1996; Romero et al, 1996a; Hjorth, 1996; Hjorth and Auerbach, 1996; Dawson and Nguyen, 2000) or more selective 5-HT1A receptor antagonist compounds such as WAY100635 (Romero et al, 1996a, 1996b; Hjorth et al, 1997; Dawson and Nguyen, 1998). A combination of 5-HT1A and 5-HT1B (or 5-HT1B/D) receptor antagonists and SSRIs (Sharp et al, 1997; Gobert et al, 2000; Dawson and Nguyen, 2000) augments extracellular 5-HT even further. SB-649915-B significantly increased 5-HT in the frontal cortex of rats and guinea pigs after acute administration while, in contrast, an acute dose of paroxetine had no effect (Hughes et al, 2007). Despite the increase in 5-HT levels following acute SB-649915-B administration, the behavioral effect in the SI paradigm was not observed until day 7 of dosing. Hence, presumably there are downstream effects as a result of the increase in extracellular 5-HT that mediate this response.
It has previously been reported that chronic administration of SSRIs, including paroxetine, to rodents (Kennett et al, 1994b; Maj et al, 1996; Yamauchi et al, 2004) and man (Zohar et al, 1988; Hollander et al, 1991; Quested et al, 1997) cause 5-HT2C receptor desensitization. In the SI test, the anxiogenic effects of the 5-HT2C receptor agonist mCPP (see Kennett et al, 1989) are inhibited by the coadministration of WAY100635 and fluoxetine for 7 days (Bristow et al, 2000) while no effect is seen with an SSRI alone. Moreover, 5-HT2C receptor antagonists and inverse agonists have been shown to be acutely anxiolytic in rodent models including the SI (Kennett et al, 1997, 2000; Wood et al, 2001). Therefore, it is possible that the acute increase in 5-HT seen following SB-649915-B (Hughes et al, 2007) results in a rapid desensitization of 5-HT2C receptors which are, at least in part, mediating the effect. However, further work is needed to investigate this hypothesis.
More long-term neuronal plastic processes such as neurogenesis have been implicated in antidepressant-mediated clinical efficacy (Duman, 2004). Furthermore, evidence suggests that the effect of antidepressants on neurogenesis is observed after chronic, but not acute administration (Malberg et al, 2000; Duman, 2004). The effects of increased 5-HT on cell proliferation are thought to be, at least in part, mediated via agonism of 5-HT1A receptors in the hippocampus (Gould, 1999). Indeed, 5-HT1A receptor antagonists have been shown to reduce cell proliferation in the hippocampus (Radley and Jacobs, 2002). It could be of concern that the 5-HT1A receptor antagonist properties of SB-649915-B may impact neurogenesis. Although this has not been specifically investigated, at 1.0 and 3.0 mg/kg, the postsynaptic 5-HT1A receptors in the hippocampus are, potentially, not saturated by SB-649915-B, as determined by an inability to block fully 8-OH-DPAT-induced hyperactivity (Hughes et al, 2007). The extracellular increase in 5-HT (Hughes et al, 2007) seen at these doses may be acting at available 5-HT1A receptors, inducing neurogenesis and thereby mediating the anxiolytic effect in the SI. This is supported by the lack of effect of 7.5 mg/kg at this early time point, assuming this dose completely blocks pre- and postsynaptic 5-HT1A receptors as seen at 10 mg/kg (Hughes et al, 2007). After 21 days administration, there is a significant increase in SI at 7.5 mg/kg indicating that, if neurogenesis is playing a role in the anxiolytic behavior, 5-HT1A receptor activation may have occurred at this time point, and a longer period of 5-HT elevation is required to overcome the SB-649915-B-mediated competitive 5-HT1A receptor block. It could be suggested that chronic blockade of postsynaptic 5-HT1A receptors will induce an increase in receptor population or sensitization, however, the literature dose not support a change in 5-HT1A receptor sensitivity (Dawson et al, 2002; Hervas et al, 2001). The Duxon et al (2000) data showing an accelerated onset of effect following coadministration of paroxetine (3.0 mg/kg) and WAY100635 (0.1 mg/kg) clearly indicate that 5-HT1A receptor antagonism does not have a negative impact on the SI responses. Clinical efficacy with pindolol in combination with SSRIs may also lend some support to this hypothesis although, the level of 5-HT1A receptor blockade achieved with this combination has been questioned (Rabiner et al, 2001).
It could be proposed that the differing dosing regimen used for paroxetine and SB-649915-B (once-daily dosing of paroxetine vs thrice-daily dosing of SB-649915-B) could have contributed, at least in part, to the improvement in latency to efficacy. The SB-649915-B dosing regimen was chosen to provide an appropriate level of exposure over a 24 h period (data not shown), and the paroxetine based on previous data showing efficacy in this test (Lightowler et al, 1994). Put into context with previous data, we suggest that a higher dose of paroxetine, or increased frequency of dosing would not drive a more rapid onset of effect, as at 10 mg/kg there was no efficacy in the SI following 21 days dosing (Lightowler et al, 1994). Duxon et al, (2000) showed an accelerated onset in the SI following a combination of paroxetine and the 5-HT1A receptor antagonist WAY100635, therefore it is likely that novel pharmacology of SB-649915-B is responsible for the accelerated action and not the differences in dosing regimen used. SB-649915-B clearly shows an advantage over paroxetine with regards to onset of anxiolytic activity in the SI paradigm. Although the top (7.5 mg/kg) dose of SB-649915-B failed to demonstrate an anxiolytic effect after 7 days administration, it is noteworthy that the efficacy of paroxetine alone after 21 days administration in this model also displays a bell-shaped dose–response curve with only 3.0 mg/kg (but not 1.0 or 10 mg/kg) significantly increasing SI time (Lightowler et al, 1994). These authors are not aware of any SI studies where administration of SSRIs beyond 21 days dosing has been investigated, but it is possible that, like SB-649915-B, prolonged dosing of paroxetine yields an anxiolytic effect at doses that have had no effect at earlier time points.
In conclusion, SB-649915-B is a novel compound showing SSRI activity together with selective 5-HT1A/B receptor blockade in a single molecule (Atkinson et al, 2005; Scott et al, 2006). The anxiolytic activity of this compound is shown in rodent and primate tests which also detect the activity of SSRIs after acute dosing. After chronic administration SB-649915-B clearly shows an advantage over paroxetine in reducing the latency of onset to anxiolytic activity from 21 to 7 days in the SI paradigm, a suggested surrogate marker of onset to SSRI activity in the clinic. The clear anxiolytic effect at both 7 and 21 days indicates a lack of tolerance to the compound. These data indicate that the augmentation of an SSRI with 5-HT1A/B receptor antagonism in a single compound reduces the latency to anxiolytic effect compared to a selective SSRI alone and this may be of therapeutic benefit.
References
Artigas F (1993). 5-HT and antidepressants: new views from microdialysis studies. Trends Pharmacol Sci 14: 262.
Artigas F, Perez V, Alvarez E (1994). Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry 51: 48–51.
Atkinson PJ, Bromidge S, Duxon MS, Gaster L, Hadley MS, Hammond B et al (2005). 3,4-Dihydro-2H-benzoxazinones are 5-HT1A receptor antagonists with potent 5-HT reuptake inhibitory activity. Bioorg Med Chem Lett 15: 737–741.
Ballesteros J, Callado LF (2004). Effectiveness of pindolol plus serotonin uptake inhibitors in depression: a meta-analysis of early and late outcomes from randomised controlled studies. J Affect Disord 79: 137–147.
Berman RM, Anand A, Cappiello A, Miller HL, Hu XS, Oren DA et al (1999). The use of pindolol with fluoxetine in the treatment of major depression: final results from a double-blind, placebo-controlled trial. Biol Psychiatry 45: 1170–1177.
Berman RM, Darnell AM, Miller HL, Anand A, Charney DS (1997). Effect of pindolol in hastening response to fluoxetine in the treatment of major depression: a double-blind, placebo-controlled trial. Am J Psychiatry 154: 37–43.
Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F (1999). Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience 92: 327–341.
Blier P, Bergeron R (1995). Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. J Clin Psychopharmacol 15: 217–222.
Blier P, Bouchard C (1994). Modulation of 5-HT release in the guinea-pig brain following long term administration of antidepressant drugs. Br J Pharmacol 113: 485–495.
Blier P, de Montigny C, Chaput Y (1987). Modifications of the serotonin system by antidepressant treatments: implications for the therapeutic response in major depression. J Clin Psychopharmacol 7: 24S–35S.
Bordet R, Thomas P, Dupuis B (1998). Effect of pindolol with selected antidepressant drugs in the treatment of major depression: intermediate analysis of a double blind, placebo-controlled trial. Am J Psychiatry 155: 1346–1351.
Borsini F, Podhorna J, Marazziti D (2002). Do animal models of anxiety predicit anxiolytic-like effects of anti-depressants? Psychopharmacology 163: 121–141.
Boulenguez P, Rawlins JN, Chauveau J, Joseph MH, Mitchell SN, Gray JA (1996). Modulation of dopamine release in the nucleus accumbens by 5-HT1B agonists: involvement of the hippocampo-accumbens pathway. Neuropharmacology 35: 1521–1529.
Bristow LJ, O'Connor D, Watts R, Duxon MS, Hutson PH (2000). Evidence for accelerated desensitization of 5-HT2C receptors following combined treatment with fluoxetine and the 5-HT1A receptor antagonist, WAY 100, 635, in the rat. Neuropharmacology 39: 1222–1236.
Consolo S, Arnaboldi S, Ramponi S, Nannini L, Ladinsky H, Baldi G (1996). Endogenous serotonin facilitates in vivo acetylcholine release in rat frontal cortex through 5-HT 1B receptors. J Pharmacol Exp Ther 277: 823–830.
Costall B, Domeney AM, Gerrard PA, Kelly ME, Naylor RJ (1988a). Zaclopride: anxiolytic profile in rodent and primate models of anxiety. J Pharm Pharmacol 40: 302–305.
Costall B, Domeney AM, Gerrard PA, Naylor RJ (1988b). A primate model for the assessment of anxiolytic drug action. Br J Pharmacol 95 (suppl): 670P.
Dawson LA, Nguyen HQ (1998). Effects of 5-HT1A receptor antagonists on fluoxetine-induced changes in serotonin in rat frontal cortex. Eur J Pharmacol 345: 41–46.
Dawson LA, Nguyen HQ (2000). The role of 5-HT1A and 5-HT1B/1D receptors on the modulation of acute fluoxetine-induced changes in extracellular 5-HT: the mechanism of action of (±) pindolol. Neuropharmacology 39: 1044–1052.
Dawson LA, Nguyen HQ, Smith DL, Schechter LE (2002). Effect of chronic fluoxetine and WAY-100635 treatment on serotonergic neurotransmission in the frontal cortex. J Psychopharmacol 16: 145–152.
Draper NR, Smith H (1966). Applied Regression Analysis. John Wiley and Sons: New York, NY.
Dreshfield LJ, Wong DT, Perry KW, Engleman EA (1996). Enhancement of fluoxetine-dependent serotonin (5-HT) levels by (−) pindolol, an antagonist at 5-HT1A receptors. Neurochem Res 21: 557–562.
Duman RS (2004). Depression: a case of neuronal life and death? Biol Psychiatry 56: 140–145.
Duxon MS, Starr KR, Upton N (2000). Latency to paroxetine-induced anxiolysis in the rat is reduced by co-adminstration of the 5-HT1A receptor antagonist WAY100635. Br J Pharmacol 130: 1713–1719.
File SE (1980). The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods 2: 219–238.
File SE, Hyde JR (1978). Can social interaction be used to measure anxiety? Br J Pharmacol 62: 19–24.
File SE, Hyde JR (1979). A test of anxiety that distinguishes between the actions of benzodiazepines and those of other minor tranquilisers and of stimulants. Pharmacol Biochem Behav 11: 65–69.
Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE et al (1996). Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res 73: 337–353.
Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y et al (1995). A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur J Pharmacol 281: 81–88.
Gardner CR (1985). Distress vocalization in rat pups: a simple screening method for anxiolytic drugs. J Pharmacol Methods 14: 181–187.
Gartside SE, Umbers V, Hajos M, Sharp T (1995). Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol 115: 1064–1070.
Gobert A, Dekeyne A, Millan MJ (2000). The ability of WAY100, 635 to potentiate the neurochemical and functional actions of fluoxetine is enhanced by co-administration of SB224, 289, but not BRL15572. Neuropharmacology 39: 1608–1616.
Gobert A, Rivet J-M, Cistarelli L, Millan MJ (1997). Potentiation of the fluoxetine-induced increase in dialysate levels of serotonin (5-HT) in the frontal cortex of freely moving rats by combined blockade of 5-HT1A and 5-HT1B receptors with WAY100, 635 and GR 127, 935. J Neurochem 68: 1159–1163.
Gould E (1999). Serotonin and hippocampal neurogenesis. Neuropsychopharmacology 21: 46S–51S.
Griebel G, Blanchard DC, Agnes RS, Blanchard RJ (1995). Differential modulation of antipredator defensive behavior in Swiss-Webster mice following acute or chronic administration of imipramine and fluoxetine. Psychopharmacology 120: 57–66.
Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR (1994). Acute and chronic treatment with 5-HT reuptake inhibitors differentially modulate emotional responses in anxiety models in rodents. Psychopharmacology 113: 463–470.
Hajos-Korcsok E, McQuade R, Sharp T (1999). Influence of 5-HT1A receptors on central noradrenergic activity: microdialysis studies using (+/–)-MDL 73005EF and its enantiomers. Neuropharmacology 38: 299–306.
Hensler JG, Kovacich GB, Frazer A (1991). A quantitative autoradiographic study of serotonin1A receptor regulation. Neuropsychopharmacology 4: 131–144.
Hervas I, Vilaro MT, Romero L, Scorza CM, Mengod G, Artigas F (2001). Desensitisation of 5-HT1A autoreceptors by a low chronic fluoxetine dose effect of the concurrent administration of WAY-100635. Neuropsychopharmacology 24: 11–20.
Hjorth S (1996). Pindolol, but not buspirone, potentiates the citalopram-induced rise in extracellular 5-hydroxytryptamine. Eur J Pharmacol 303: 183–186.
Hjorth S, Auerbach SB (1996). 5-HT1A autoreceptors and the mode of action of selective serotonin reuptake inhibitors (SSRI). Behav Brain Res 73: 281–283.
Hjorth S, Suchowski CS, Galloway MP (1995). Evidence for 5-HT autoreceptor-mediated, nerve impulse-dependent, control of 5-HT synthesis in the rat brain. Synapse 19: 170–176.
Hjorth S, Westlin D, Bengtsson HJ (1997). WAY100635-induced augmentation of the 5-HT-elevating action of citalopram: relative importance of the dose of the 5-HT1A (auto) receptor blocker versus that of the 5-HT reuptake inhibitor. Neuropharmacology 36: 461–465.
Hollander E, Decaria C, Gully R, Nitescu A, Suclow RF, Gorman JM et al (1991). Effects of chronic fluoxetine treatment on behavioral and neuroendocrine responses to meta-chlorophenylpiperazine in obsessive-compulsive disorder. Psychiatry Res 36: 1–17.
Hoyer D, Middlemiss DN (1989). The pharmacology of the terminal 5-HT autoreceptors in mammalian brain: evidence for species differences. Trends Pharmacol Sci 10: 130–132.
Hughes ZA, Starr KR, Scott CM, Hill M, Atkinson PJ, Newson MJ et al (2007). Simultaneous blockade of 5-HT1A/B receptors and 5-HT transporters results in acute increases in extracellular 5-HT in both rats and guinea pigs: in vivo characterization of the novel 5-HT1A/B receptor/5-HT transport inhibitor SB-649915-B. Psychopharmacology (In press on-line).
Iyer RN, Bradberry CW (1996). Serotonin-mediated increase in prefrontal cortex dopamine release: pharmacological characterization. J Pharmacol Exp Ther 277: 40–47.
Kennett GA, Lightowler S, De Biasi V, Stevens NC, Wood MD, Tulloch IF et al (1994b). Effect of chronic administration of selective 5-hydroxytryptamine and noradrenaline uptake inhibitors on a putative index of 5-HT2C/2B receptor function. Neuropharmacology 23: 1581–1588.
Kennett GA, Lightowler S, Trail B, Bright F, Bromidge S (2000). Effects of RO 60 0175, a 5-HT(2C) receptor agonist, in three animal models of anxiety. Eur J Pharmacol 387: 197–204.
Kennett GA, Whitton P, Shah K, Curzon G (1989). Anxiogenic-like effects of mCPP and TFMPP in animal models are opposed by 5-HT1C receptor antagonists. Eur J Pharmacol 164: 445–454.
Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V et al (1997). SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36: 609–620.
Koyama T, Nakajima Y, Fujii T, Kawashima K (1999). Enhancement of cortical and hippocampal cholinergic neurotransmission through 5-HT1A receptor-mediated pathways by BAY × 3702 in freely moving rats. Neurosci Lett 265: 33–36.
Le Poul E, Laaris N, Doucet E, Laporte A, Hamon M, Lanfumey L (1995). Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn-Schmiedebergs Arch Pharmacol 352: 141–148.
Lightowler S, Kennett GA, Williamson IJ, Blackburn TP, Tulloch IF (1994). Anxiolytic-like effect of paroxetine in a rat social interaction test. Pharmacol Biochem Behav 49: 281–285.
Maes M, Libbrecht I, Van Hunsel F, Campens D, Meltzer HY (1999). Pindolol and mianserin augment the antidepressant activity of fluoxetine in hospitalized major depressed patients, including those with treatment resistance. J Clin Psychopharmacol 19: 177–182.
Maj J, Bijak M, Dziedzicka M, Rogoz R, Rogoz Z, Skuza G et al (1996). The effects of paroxetine given repeatedly on the 5-HT receptor subpopulations in the rat brain. Psychopharmacology 127: 73–82.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110.
Marcoli M, Maura G, Munari C, Ruelle A, Raiteri M (1999). Pharmacological diversity between native human 5-HT1B and 5-HT1D receptors sited on different neurons and involved in different functions. Br J Pharmacol 126: 607–612.
McAskill R, Mir S, Taylor D (1998). Pindolol augmentation of antidepressant therapy. B J Psychiatry 173: 203–208.
Mongeau R, Blier P, De Montigny C (1997). The serotonergic and noradrenergic systems of the hippocampus: their interactions and the effects of antidepressant treatments. Brain Res Rev 23: 145–195.
Nakai K, Fujii T, Fujimoto K, Suzuki T, Kawashima K (1998). Effect of WAY-100135 on the hippocampal acetylcholine release potentiated by 8-OH-DPAT, a serotonin1A receptor agonist, in normal and p-chlorophenylalanine-treated rats as measured by in vivo microdialysis. Neurosci Res 31: 23–29.
Newman ME, Shalom G, Ran A, Gur E, Van De Kar LD (2004). Chronic fluoxetine-induced desensitization of 5-HT1A and 5-HT1B autoreceptors: regional differences and effects of WAY-100635. Eur J Pharmacol 486: 25–30.
Nomikos GG, Arborelius L, Hook BB, Hacksell U, Svensson TH (1996). The 5-HT1A receptor antagonist (S)-UH-301 decreases dopamine release in the rat nucleus accumbens and striatum. J Neural Transm 103: 541–554.
Nutt DJ (2005). Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr 10: 49–56.
Olivier B, Molewijk HE, Van Der Heydden JA, Van Oorschot R, Ronken E, Mos J et al (1998). Ultrasonic vocalizations in rat pups: effects of serotonergic ligands. Neurosci Biobehav Rev 23: 215–227.
Quested DJ, Sargent PA, Cowen PJ (1997). SSRI treatment decreases prolactin and hyperthermic responses to mCPP. Psychopharmacology 133: 305–308.
Rabiner EA, Bhagwagar Z, Gunn RN, Sargent PA, Bench CJ, Cowen PJ et al (2001). Pindolol augmentation of selective serotonin reuptake inhibitors: PET evidence that the dose used in clinical trials is too low. Am J Psychiatry 158: 2080–2082.
Radley JJ, Jacobs BL (2002). 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res 955: 264–267.
Roberts C, Boyd DF, Middlemiss DN, Routledge C (1999). Enhancement of 5-HT1B and 5-HT1D receptor antagonist effects on extracellular 5-HT levels in the guinea-pig brain following concurrent 5-HT1A or 5-HT re-uptake site blockade. Neuropharmacology 38: 1409–1419.
Rollema H, Clarke T, Sprouse JS, Schultz DW (1996). Combined administration of a 5-HT1D antagonist and 5-HT reuptake inhibitor synergistically increases 5-HT release in guinea-pig hypothalamus in vivo. J Neurochem 67: 2204–2207.
Romero L, Bel N, Artigas F, De Montigny C, Blier P (1996a). Effects of pindolol on the function of pre- and postsynaptic 5-HT1A receptors: in vivo microdialysis and electrophysiological studies in the rat brain. Neuropsychopharmacology 15: 349–360.
Romero L, Hervas I, Artigas F (1996b). The 5-HT1A antagonist WAY-100635 selectively potentiates the presynaptic effects of serotonergic antidepressants in the rat brain. Neurosci Lett 219: 123–126.
Schechter LE, Dawson LA, Harder JA (2002). The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer's disease. Curr Pharmaceut Des 8: 139–145.
Scott CM, Soffin EM, Hill M, Atkinson PJ, Langmead CJ, Wren PB et al (2006). SB-649915, a novel, potent 5-HT1A and 5-HT1B autoreceptor antagonist and 5-HT re-uptake inhibitor in native tissue. Eur J Pharmacol 536: 54–61.
Segrave R, Nathan PJ (2005). Pindolol augmentation of selective serotonin reuptake inhibitors: accounting for the variability of results of placebo-controlled double-blind studies in patients with major depression. Hum Psychopharmacol Clin Exp 20: 163–174.
Shalom G, Gur E, Van DeKar LD, Newman ME (2004). Repeated administration of the 5-HT1B receptor antagonist SB-224289 blocks the desensitization of 5-HT1B autoreceptors induced by fluoxetine. Naunyn-Schmiedebergs Arch Pharmacol 370: 84–90.
Sharp T, Umbers V, Gartside SE (1997). Effect of a selective 5-HT reuptake inhibitor in combination with 5-HT1A and 5-HT1B receptor antagonists on extracellular 5-HT in rat frontal cortex in vivo. Br J Pharmacol 121: 941–946.
Stein MB, Sareen J, Hami S, Chao J (2001). Pindolol potentiation of paroxetine for generalized social phobia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry 158: 1725–1727.
Thomas DR, Nelson DR, Johnson AM (1987). Biochemical effects of the antidepressant paroxetine, a specific 5-hydroxytryptamine uptake inhibitor. Psychopharmacology 93: 193–200.
To CT, Anheuer ZE, Bagdy G (1999). Effects of acute and chronic fluoxetine treatment of CRH-induced anxiety. Neuroreport 10: 553–555.
To CT, Bagdy G (1999). Anxiogenic effect of central CCK administration is attenuated by chronic fluoxetine or ipsapirone treatment. Neuropharmacology 38: 279–282.
Wood MD, Reavill C, Trail B, Wilson A, Stean T, Kennett GA et al (2001). SB-243213; a selective 5-HT2C receptor inverse agonist with improved anxiolytic profile: lack of tolerance and withdrawal anxiety. Neuropharmacology 41: 186–199.
Yamauchi M, Tatebayashi T, Nagase K, Kojima M, Imanishi T (2004). Chronic treatment with fluvoxamine desensitizes 5-HT2C receptor-mediated hypolocomotion in rats. Pharmacol Biochem Behav 78: 683–689.
Zanardi R, Artigas F, Franchini l, Sforzini L, Gasperini M, Smeraldi E et al (1997). How long should pindolol be associated with paroxetine to improve anti-depreassant response? J Clin Psychopharmacol 17: 446–450.
Zohar J, Insel TR, Zohar-Kadouch RC, Hill JL, Murphy DL (1988). Serotonergic responsivity in obsessive-compulsive disorder. Effects of chronic clomipramine treatment. Arch Gen Psychiatry 45: 167–172.
Acknowledgements
We acknowledge the assistance of Dr Declan Jones in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Starr, K., Price, G., Watson, J. et al. SB-649915-B, a Novel 5-HT1A/B Autoreceptor Antagonist and Serotonin Reuptake Inhibitor, is Anxiolytic and Displays Fast Onset Activity in the Rat High Light Social Interaction Test. Neuropsychopharmacol 32, 2163–2172 (2007). https://doi.org/10.1038/sj.npp.1301341
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301341
Keywords
This article is cited by
-
Reduced isolation-induced pup ultrasonic communication in mouse pups lacking brain serotonin
Molecular Autism (2015)
-
Anticonvulsant and anxiolytic-like effects of compounds isolated from Polygala sabulosa (Polygalaceae) in rodents: in vitro and in vivo interactions with benzodiazepine binding sites
Psychopharmacology (2008)
-
Simultaneous blockade of 5-HT1A/B receptors and 5-HT transporters results in acute increases in extracellular 5-HT in both rats and guinea pigs: in vivo characterization of the novel 5-HT1A/B receptor antagonist/5-HT transport inhibitor SB-649915-B
Psychopharmacology (2007)