Abstract
The purpose of this study was to determine whether schizophrenia was associated with alterations in alcohol response that might explain the elevated risk for AUDs in this population. In a randomized, double-blind, placebo-controlled, counter-balanced 3 test day laboratory study, the effects of alcohol were compared in 23 subjects with schizophrenia (without any previous alcohol use disorder (AUD) but with some alcohol exposure) and in 14 healthy subjects matched for age, gender, education, and lifetime exposure to alcohol. Standard alcohol drinks in a scheduled design were administered to produce blood alcohol levels of 0, 0.02–0.04 mg%, or 0.06–0.08 mg%. Schizophrenia symptoms, perceptual alterations, stimulant and depressant subjective effects of alcohol, and ‘high’ were measured before alcohol administration and at several post-drug time points. Verbal learning and recall, vigilance and distractibility, and motor function were assessed once per test day. Relative to healthy subjects, subjects with schizophrenia reported greater euphoria and stimulatory effects in response to alcohol. Alcohol produced small transient increases in positive psychotic symptoms and perceptual alterations without affecting negative symptoms. Alcohol also impaired several aspects of immediate and delayed recall, and vigilance, and distractibility. Schizophrenia patients showed increased euphoric and stimulatory responses to alcohol. These exaggerated positive responses to alcohol doses may contribute to the increased risk for AUDs associated with schizophrenia. The absence of ‘beneficial’ effects of alcohol does not support a self-medication hypothesis of alcohol use in schizophrenia.
Similar content being viewed by others
INTRODUCTION
There are higher rates of alcohol use disorders (AUD) in schizophrenia than in the general population (Drake et al, 2000) and AUDs have a negative impact on the course and outcome of schizophrenia (Drake et al, 1989, 1996; Cuffel and Chase 1994). However, little is known about why there is an elevated risk for AUDs in this population. The degree of subjective response to alcohol is a substantial contributor to the risk for problematic drinking. Individuals who experience enhanced positive responses or reduced negative responses to alcohol are at increased risk for subsequent development of AUD (Schuckit et al, 1996; Erblich et al, 2001). Some reports suggest that schizophrenia patients use alcohol to ‘self-medicate’ psychotic symptoms, subjective distress, insomnia, social anxiety, or medication side effects, whereas other studies suggest that subjects with schizophrenia use alcohol for its stimulatory or euphoric effects (Noordsy et al, 1991; Chambers et al, 2001; Green, 2005). This study was undertaken to (1) characterize the dose-related effects of alcohol in schizophrenia patients and (2) to compare responses to alcohol in patients and healthy subjects, in order to find explanations for the elevated rates of AUDs in schizophrenia. Alcohol was hypothesized to produce euphoric and anxiolytic effects and to alleviate some symptoms and medication side effects associated with schizophrenia.
METHODS
The study was conducted at the Neurobiological Studies Unit (VA Connecticut Healthcare System, West Haven, CT) with the approval of the Institutional Review Boards of Yale University School of Medicine and VA Connecticut Healthcare System and in accordance with the Helsinki Declaration of 1975. The study was a double-blind, placebo-controlled comparison of the effects of varying blood alcohol levels (BALs) in subjects with schizophrenia versus healthy control subjects. Subjects were recruited by advertisements and by word of mouth and were paid for their participation at $100 per test day.
Consent Process
Subjects were cautioned that the study (1) was not a treatment, (2) was not intended to improve schizophrenia symptoms, (3) carried the possible risk of symptoms worsening, relapse, and hospitalization, and (4) was not a sanction to use alcohol. Subjects were required to pass a questionnaire about the study risks and benefits. Collaterals were involved in the consent process, inclusion was contingent on the clinician's approval, and an ombudsperson (clinician) was appointed to serve subjects.
Subjects and Screening
Stable, medication-adherent, schizophrenia, and schizoaffective disorder subjects 18–65 years without any previous AUD were compared to age, sex, and education-matched healthy control subjects. Subjects with a remitted AUD, were excluded out of concern that study participation might increase the risk of relapse. This study of subjects with schizophrenia but without an AUD was a prelude to studying subjects with an AUD. Patients deemed unstable, as defined by recent (3 months) or current hospitalization, or recent (3 months) homicidality, suicidality, or grave disability, were excluded. Subjects with a lifetime diagnosis of substance dependence, other than nicotine, and current treatment drugs that could interfere with alcohol effects (eg, benzodiazepines and anticonvulsants) were excluded from the study. Alcohol-naïve individuals were excluded for ethical reasons.
Screening
All subjects received a medical evaluation including medical history and physical examination, laboratory tests (hematology, serum chemistry profile, liver and thyroid studies, urinalysis, urine toxicology, VDRL, urine pregnancy test (for females), and EKG. Diagnosis was confirmed by interview with a research psychiatrist, a structured psychiatric interview for DSM-III-RP (Spitzer et al, 1990), chart review and by contact with an informant and primary clinician. Alcohol consumption history over the preceding 3 months was determined using a validated calendar completion technique (Sobell et al, 1992). In schizophrenia patients, alcohol use data was further determined by chart review, breathalyzer, clinician interview, and laboratory biomarkers suggestive of alcohol use (mean corpuscular volume and liver enzymes). Healthy subjects underwent a Structured Clinical Interview for DSM-IIIR-NP (non-patient version) (Spitzer et al, 1990) and clinical interview by a research psychiatrist.
General Study Design
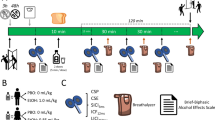
Subjects completed 3 test days separated by at least 3 days in a randomized, counterbalanced order within a 2-week period. Subjects received high (0.8 g/kg), low (0.4 g/kg body weight), and placebo dose alcohol per oral to reach peak BAL of 0.06–0.08, 0.02–0.04%, and 0, respectively. These methods were in compliance with the National Advisory Council on Alcohol Abuse and Alcoholism guidelines for alcohol administration (Lawson et al, 1980). These BALs are socially relevant and produce stimulant and sedative effects in most social drinkers over the course of the blood alcohol curve (Holdstock and de Wit, 1998). The relevant dose of alcohol was mixed with ginger ale to make up 120 ml and this mixture was divided into four 30 ml drinks. Subjects received three drinks over 40 min (0, 20, and 40 min). All subjects received a placebo drink at 60 min unless the target BAL range had not been attained by that time point, in which case an active booster drink was administered. A research pharmacist not involved in data collection monitored the BAL and made the decision to administer the active or placebo booster dose without unblinding staff involved in data collection. For the placebo condition, the first drink was covered by a trace amount (5 ml) of alcohol on the surface, to maintain the blind/placebo deception; the latter was confirmed by post-experimental questionnaire and ratings of subjective intoxication. Female subjects received only 85% of the dose administered to male subjects.
Schedule of test days
Subjects were instructed to fast overnight and testing began in the morning (08:00). Cigarette smoking was not permitted from the time the intravenous lines were inserted until after the cognitive testing was completed. The sequence of procedures is described in Table 1.
Outcome measures
Alcohol levels were estimated both indirectly by breathalyzer (IR-3000; Intoximeters Inc.) and directly assayed from samples of venous blood using enzymatic methods (Diagnostic Chemicals Ltd PE, Canada). Positive and negative symptoms were measured using the four-key positive symptom (conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content) and the three-key negative (blunted affect, emotional withdrawal, and motor retardation) subscales of the Brief Psychiatric Rating Scale (BPRS), respectively (Overall and Gorham, 1962). Perceptual alterations were measured using the eight clinician-rated items (0=not at all, 4=extremely) of the Clinician Administered Dissociative Symptoms Scale (CADSS) (Bremner et al, 1998). The state of euphoria described as ‘high’ was measured using a Visual Analog Scale; subjects were asked to score the perceived intensity of the feeling state (high) at that moment on a 100 mm line (0=not at all, 100=extremely). Effects associated with alcohol were captured using the Biphasic Alcohol Effects Scale (BAES) a test that measures the stimulatory (‘elated’, ‘energized’, ‘excited’, ‘stimulated’, ‘talkative’, ‘up’, and ‘vigorous’) and sedating (‘down,’ ‘heavy head,’ ‘inactive’, ‘sedated’, ‘slow thoughts’, ‘sluggish’, and ‘difficulty concentrating’) effects of alcohol on a scale of 0 (not at all) to 10 (extremely) (Martin et al, 1993). As alcohol was expected to interfere with self-report, well after the effects of alcohol had worn off (=170 min), subjects were asked to report on peak effects experienced earlier in the study (retrospective rating). Dyskinesia, akathisia, and parkinsonism were also evaluated but data are not reported because of the low rates of these side effects in this sample.
Learning and recall were measured using the Hopkins Verbal Learning Test (HVLT) (Brandt et al, 1992). The test consists of three consecutive trials of immediate free recall of a 12-item, semantically categorized list, followed 30 min later by testing of delayed free recall, cued recall, and recognition recall. Different but equivalent versions of the test were administered on the 3 test days. Vigilance and distractibility to visual stimuli were measured using a continuous performance task (Gordon, 1986) in which subjects attended to numbers presented sequentially on a screen. The subject pushed a button to signal when a ‘9’ was preceded by a ‘1’. The distractibility task was identical to the vigilance task with the exception that numbers were presented sequentially in three contiguous columns. Subjects were instructed to attend to the middle column and ignore the outer two columns.
The same research assistant rated all 3 test days for each subject, and schizophrenia patients and healthy subjects were rated by the same pool of raters. Monthly inter-rater reliability sessions were conducted and intraclass correlations for the BPRS and CADSS were consistently greater than 0.85.
At the end of each test day, before discharge subjects had to pass a field sobriety test.
The study was amended to include prospective assessments (chart review and telephone or in person interview) at 1, 3, and 6 months after the last test session to detect the emergence of any new medical or psychiatric symptoms and any changes in the course of schizophrenia.
Statistical Analysis
All analyses were performed in SAS version 9.2 (SAS Institute, Cary, North Carolina). The change from baseline data was assessed for normality before analysis according to normal probability plots and Kolmogorov–Smirnov test statistics. All data from subjects who initiated the study, including dropouts, was analyzed. The distributions of the behavioral data were skewed in both groups. The change from baseline scores were approximately normally distributed in the patient group, but were still skewed in the healthy subjects. Therefore, parametric mixed model was applied in the analysis of change from baseline scores in the patient group and a non-parametric model was used in both the analysis of change from baseline scores in the healthy control and in the comparison between healthy subjects and patients. The effects of alcohol on control subjects are reported only in the context of group comparisons with schizophrenia patients. For parsimony retrospective data are reported only when they conflict with data collected at the +30 time point.
BPRS, CADSS, VAS, and BAES data were analyzed using SAS PROC MIXED according to mixed-effects models with dose, time, and dose by time interaction as fixed effects and structured variance–covariance pattern matrix. The best-fitting variance–covariance matrix according to the Akaike Information Criterion was selected. When a significant dose by time interaction was observed, follow-up pairwise comparisons were performed between the least squares means at the three doses at relevant time points.
A non-parametric approach (Brunner and Langer, 2002) with group as a between-subjects factor was used to analyze differences between subjects with schizophrenia and healthy subjects. For all repeatedly measured outcomes within a test day (eg, PANSS, CADSS, and VAS) the %F1_LD_F2 SAS macro was used, and dose and time were included as within-subject factors. For all outcomes measured only once per test day (recall, distractibility, and vigilance) the %F1_LD_F1 macro was used, and dose was included as a within-subject factor. Relative effects plots were used to interpret significant interactions and main effects. The overall α level for each hypothesis was fixed at the 0.05 level. When the dose effect was significant, follow-up pairwise comparisons between doses at relevant time points were performed. Secondary behavioral, cognitive and motor data were analyzed similar to primary outcome data. Given the potential impact of atypical antipsychotics on substance abuse in schizophrenia (Green et al, 2003), we also conducted post hoc analyses to determine whether there were any differences in responses to alcohol between patients treated with typical versus atypical antipsychotics.
RESULTS
Schizophrenia patients were comparable in age (t=1.6, df=37, p<0.13), gender, education, lifetime alcohol exposure, and last exposure to alcohol to healthy subjects, but were heavier (t=1.34, df=29, p<0.05), more likely to be smokers and were all receiving stable doses of antipsychotic medications (Table 2). There were no obvious differences between dropouts and completers. The reasons for dropout included scheduling conflicts (n=1), and withdrawal of consent (n=3). As the effects of alcohol in healthy humans are well known, for parsimony, only the effects of alcohol in schizophrenia patients are reported.
BAL
BALs estimated by breathalyzer and confirmed in venous samples demonstrated that subjects had reached targeted levels (0, 0.02–0.04, and 0.06–0.08%), without any differences between healthy subjects and subjects with schizophrenia.
Behavioral Measures
‘High’
Alcohol increased self-reported ‘high’ measured by the Visual Analog Scale in schizophrenia patients in a dose-related manner (Figure 1); the dose (F2,216=20.61, p<0.0001), time (F3,216=21.66, p<0.0001) and dose by time (F6,216=5.31, p<0.0001) effects were all significant.
Means and SEM showing the time course and dose-related effects of alcohol on ‘high’ by a Visual Analog Scale (0=not at all, to 100=extremely). Data from subjects with schizophrenia are on the left whereas data from healthy subjects is on the right. High dose=BAL of 0.06–0.08%, low dose=BAL of 0.02–0.04%, and placebo=trace. Group by dose: ATS(1.73)=4.8, p=0.012.
Group comparison
Although there were no baseline group differences, schizophrenia patients reported greater and longer lasting dose-related increases in alcohol induced ‘high’ relative to healthy subjects (group by dose: Anova Type Statistic (ATS) (1.73)=4.8, p=0.012; group by time: ATS(2.46)=17.60, p<0.0001). However, the interaction of group by dose by time was not significant (ATS(4.84)=1.29, p=0.26). Consistent with the real time data, the retrospective assessment of alcohol induced ‘high’ also showed greater effects in schizophrenia patients (ATS(1)=4.51, p=0.034).
Positive symptoms
Although BPRS four-key positive symptom subscale scores decreased over time (time effect: F3,57.8=4.94, p=0.004), alcohol had no significant dose (F2,41.8=0.16, p=0.85) and dose by time interactive (F6,75.6=1.19, p=0.32) effects in schizophrenia patients (Figure 2). However, alcohol trended to increase retrospectively assessed positive symptoms (dose effect: F2,29.9=2.57, p=0.09), with the high dose increasing BPRS four-key positive symptom subscale scores by 1.5(±0.52) points.
Means and SEM showing the time course and dose-related effects of alcohol on (1) positive symptoms of psychosis (left panel) measured by the four-key positive symptoms subscale of the BPRS and (2) perceptual alterations (right panel) measured by the CADSS in subjects with schizophrenia. Positive symptoms of psychosis include items for conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content that are scored from 1 (not present) to 7 (extremely severe). The ranges of scores on the positive subscale is from 6 to 42. The CADSS consists of eight clinician-rated items that are scored from 0 (not at all) to 4 (extremely). The ranges of scores on the positive subscale is from 8 to 32. High dose=BAL of 0.06–0.08%, low dose=BAL of 0.02–0.04%, and placebo=trace.
Group comparison
Schizophrenia patients had greater positive symptoms than controls at baseline (ATS(1)=123.8, p=0.0001), none of the group interactive effects (group by time, or group by dose by time) were significant.
Negative symptoms
Alcohol had no significant dose effects (F2,36.6=0.96, p=0.39) or dose × time (F6,75.7=1.67, p=0.14) interactive effects on BPRS three-key negative symptom subscale scores in schizophrenia patients. However, negative symptoms did decrease over time (time effect: F3,57.9=7.79, p=0.0002).
Group comparison
Schizophrenia patients had greater negative symptoms than controls at baseline (ATS(1)=19.247, p=0.00001), none of the group interactive effects (group by time, or group by dose by time) were significant.
Clinician-rated perceptual alterations
Alcohol transiently increased scores of the CADSS clinician-rated subscale in schizophrenia patients; the dose by time interaction (F4,93.8=2.60, p=0.04) and time effects (F2,85.4=12.27, p<0.0001) were statistically significant, but the dose effect was not (F2,61.4=1.92, p=0.15) (Figure 2). High dose alcohol induced a two-point increase in clinician-rated CADSS scores at the +60 time point. Subjects were rated as being ‘spaced out,’ seeming separated or detached from the test environment, having said or done something bizarre, or needing redirection.
Schizophrenia patients had greater perceptual alterations at baseline (ATS(1)=12.02, p=0.0005). Further, there was a significant group by time interaction (ATS(1.75)=5.89, p=0.004), but none of the other group interactions (group by dose, or group by dose by time) were significant.
Stimulatory effects of alcohol
Alcohol trended to increase stimulatory effects in schizophrenia patients (dose: F2,151=2.87, p=0.06 and time effect: F2,151=2.65, p=0.07), but the dose by time interaction (F4,151=1.14, p=0.34) was not significant (Figure 3).
Means and SEM showing the time course and dose-related stimulatory effects of alcohol measured by the ascending limb of the BAES in schizophrenia patients (left panel) and healthy subjects (right panel). The stimulant scale score of the BAES is the sum of scores for the seven adjectives ‘elated’, ‘energized’, ‘excited’, ‘stimulated’, ‘talkative’, ‘up’, and ‘vigorous’ each of which are scored from 0 (not at all) to10 (extremely). The range of scores are from 0 to 70. High dose=BAL of 0.06–0.08%, low dose=BAL of 0.02–0.04%, and placebo=trace. Group by dose (ATS(1.28)=5.63, p=0.01).
Group comparisons
Although there were no baseline group differences, there were significant group (ATS(1)=3.96, p=0.047) and group by dose (ATS(1.28)=5.63, p=0.01) effects such that alcohol had greater effects in schizophrenia patients relative to healthy subjects. However, none of the other group interactive effects (group by time, or group by dose by time) were significant.
Depressant effects of alcohol
Surprisingly, alcohol did not increase BAES descending limb scores significantly in schizophrenia patients (F2,148=1.52, p=0.2227). However, there was a significant time effect (F2,148=16.83, p<0.0001) and dose by time interactions that trended towards significance (F4,148=2.30, p=0.06) (Figure 4).
Means and SEM showing the time course and dose-related sedating effects of alcohol measured by the descending limb of the BAES in schizophrenia patients (left panel) and healthy subjects (right panel). The sedating scale score of the BAES is the sum of scores for the seven adjectives ‘down’, ‘heavy head’, ‘inactive’, ‘sedated’, ‘slow thoughts’, ‘sluggish’, and ‘difficulty concentrating’ each of which are scored from 0 (not at all) to 10 (extremely). The range of scores are from 0 to 70. High dose=BAL of 0.06–0.08%, low dose=BAL of 0.02–0.04%, and placebo=trace.
Group comparisons
There were no baseline group differences and none of the group interactive effects (group by time, or group by dose by time) were significant.
Immediate recall
Alcohol impaired immediate recall in a dose-dependent manner with a trend towards significance (dose: F2,38.8=2.65, p=0.08) in schizophrenia patients (Figure 5). Patients recalled more words with successive trials (F2,58=67.83, p<0.0001) and alcohol did not appear to impair learning (dose by trial interaction: F4,68.8=1.35, p=0.2615).
Means and SEM showing the dose-related effects of alcohol on immediate free recall (left panel) and delayed free recall (right panel) measured by a 12-word learning task (HVLT). Scores range from 0 to 12. In each panel, data from healthy subjects are depicted on the left whereas data from schizophrenic patient are depicted on the right. High dose=BAL of 0.06–0.08%, low dose=BAL of 0.02–0.04%, and placebo=trace.
Group differences
Although schizophrenia patients performed worse than controls (ATS(1)=17.71, p=0.00003), none of the group interactions were significant in the models comparing groups over trials.
Delayed recall
Alcohol significantly impaired delayed free recall in schizophrenia patients in a dose-dependent manner (dose: F2,38.4=9.14, p=0.0006) with significant differences between low and placebo dose (F1,37.4=7.56, p=0.009) and high and placebo dose (F1,38.6=17.65, p=0.0002) (Figure 5). Although there were significant group differences in baseline delayed recall (ATS(1)=15.8, p=0.00007) the group by dose interaction was not significant.
Vigilance
Alcohol increased the number of omissions (F2,29.9=3.75, p=0.035) and this effect was related to differences between the high and low doses (F1,30.4=5.24, p=0.029), and the high and placebo doses (F1,29.6=6.13, p=0.019). Although subjects with schizophrenia performed worse on this task than controls (group: ATS(1)=8.47, p=0.003), there were no significant group by dose interactive effects. Alcohol had no effect on the number of commissions (F2,23.7=1.42, p=0.26) and there were no significant group differences.
Distractibility
Alcohol trended towards increasing the number of omissions (F2,25.3=2.78, p=0.08) but not commissions (F2,23.7=1.39, p=0.27). Although subjects with schizophrenia had both more omissions (ATS(1)=17.284, p=0.00003) and commissions (ATS(1)=6.86, p=0.008) relative to controls, there were no significant interactive effects of group and dose on either measure.
Contrasts between Typical and Atypical Antipsychotics
There were no differences between patients treated with typical versus atypical antipsychotic for the effects of alcohol on any of the outcomes presented.
Extrapyramidal Symptoms
There were very low baseline rates of akathisia, dyskinesia, and parkinsonism and no significant effects of alcohol on any of the measures of motor function.
Safety
There were no serious adverse events associated with this study. One subject vomited on the second test day (0.06–0.08% BAL) and did not complete the third test day (0.02–0.04% BAL) owing to nausea. Another subject did not complete the third test day because research staff were unable to gain intravenous access. There were no data suggesting a negative impact of study participation on the course of illness or on alcohol use at 1, 3, and 6 months.
DISCUSSION
To our knowledge, this is the first report characterizing the dose-related effects of alcohol on the symptoms and medication side effects in schizophrenia patients, and comparing the effects of alcohol between schizophrenia patients and healthy subjects. Relative to healthy subjects, subjects with schizophrenia reported greater euphoria and stimulatory effects in response to alcohol. Alcohol produced small transient increases in positive psychotic symptoms and perceptual alterations without affecting negative symptoms. Alcohol also impaired several aspects of immediate and delayed recall, as well as vigilance, and distractibility. Further, the effects of alcohol on any of the measures was not influenced by whether subjects were receiving typical or atypical antipsychotics.
The results of this study do not support the ‘self-medication’ hypothesis of alcohol and substance use in schizophrenia (Khantzian, 1997); alcohol did not reduce any of the core symptoms of the illness similar to a related laboratory study with delta-9-tetrahydrocannabinol (D'Souza et al, 2005). However, this study excluded alcohol-abusing subjects who may arguably derive ‘benefit/s’ from alcohol. The latter are the focus of an ongoing parallel study at our center. Further, perhaps the modest levels of symptoms and motor side effects in this sample may have biased against the detection of any symptom reductions. Finally, the laboratory environment and the fact that subjects did not have control over the titration of alcohol may not reflect recreational alcohol use.
The enhanced sensitivity to the euphoric and stimulatory effects of alcohol in antipsychotic-treated schizophrenia patients cannot be explained by pharmacokinetics, and/or the capacity to accurately self-report feeling states. Further, the enhanced group sensitivity was specific to some but not all alcohol induced effects, and specific to alcohol and not other substances, for example, 9-THC, administered to a similar sample (D'Souza et al, 2005).
It is unlikely that group differences in smoking status account for the group differences in response to alcohol, as compared to non-smokers and former smokers, smokers have a diminished response to alcohol challenge (Madden et al, 1995, 2000). Further, smoking was prohibited during a significant portion of the study and as the groups were not matched for smoking, the contribution of nicotine withdrawal to the group differences remains a possibility. However, the absence of any increase in anxiety, a common symptom of nicotine withdrawal, over time on the placebo test day in individuals with schizophrenia, suggests that these subjects were not in a state of nicotine withdrawal.
The reasons for increased euphoric and stimulatory responses to alcohol observed in schizophrenia patients may be related to schizophrenia and/or treatment with antipsychotic drugs. Brain reward circuitry dysfunction in schizophrenia (Chambers et al, 2001; Green, 2005) may explain the increased sensitivity to alcohol. The known effects of dopamine (DA) D2 receptor antagonists on reward mechanisms would predict blunted rather than enhanced euphoria in antipsychotic-treated subjects with schizophrenia. Alcohol effects on DA, gamma-aminobutyric acid (GABA), N-methyl-D-Aspartate (NMDA), and serotonin receptor function play a significant role in its acute intoxicating effects reviewed in Krystal and Tabakoff (2002). Disturbances in GABA, DA, and NMDA receptor function, which have been implicated in schizophrenia (Laruelle et al, 1995; Benes and Berretta, 2001; Krystal et al, 2003), may influence the response of this population to alcohol.
Individuals at high risk for alcoholism are more sensitive to the stimulating effects of alcohol and less sensitive to its sedative effects reviewed by Newlin and Thomson (1990) and similarly, alcohol is more likely to be consumed if it is experienced as strongly rewarding and/or if it causes few negative effects (de Wit et al, 1987; Chutuape and de Wit, 1994; Duka et al, 1998). Therefore, if individuals with schizophrenia experience alcohol as being more euphoric and stimulating, as our findings show, this might predispose them to use, misuse, or abuse alcohol. However, other factors must be involved in the development of AUDs as despite altered sensitivity to alcohol effects, the individuals in this sample did not go on to develop AUDs. Further work is warranted to investigate the basis of the altered alcohol sensitivity in schizophrenia and how it might contribute to the development of AUDs in schizophrenia.
References
Benes FM, Berretta S (2001). GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25: 1–27.
Brandt J, Corwin J, Krafft L (1992). Is verbal recognition memory really different in Huntington's and Alzheimer's disease. J Clin Exp Neuropsychol 14: 773–784.
Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS et al (1998). Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Traumatic Stress 11: 125–136.
Brunner EDS, Langer F (2002). Nonparametric Analysis of Longitudinal Data in Factorial Experiments. John Wiley and Sons: New York.
Chambers RA, Krystal JH, Self DW (2001). A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry 50: 71–83.
Chutuape MA, de Wit H (1994). Relationship between subjective effects and drug preferences: ethanol and diazepam. Drug Alcohol Depend 34: 243–251.
Cuffel BJ, Chase P (1994). Remission and relapse of substance use disorders in schizophrenia. Results from a one-year prospective study. J Nerv Mental Dis 182: 342–348.
D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G et al (2005). Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry 57: 594–608.
de Wit H, Uhlenhuth EH, Pierri J, Johanson CE (1987). Individual differences in behavioral and subjective responses to alcohol. Alcohol Clin Exp Res 11: 52–59.
Drake RE, Mueser KT, Clark RE, Wallach MA (1996). The course, treatment, and outcome of substance disorder in persons with severe mental illness. Am J Orthopsychiatry 66: 42–51.
Drake RE, Osher FC, Wallach MA (1989). Alcohol use and abuse in schizophrenia. A prospective community study. J Nerv Mental Dis 177: 408–414.
Drake RE, Xie H, McHugo GJ, Green AI (2000). The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull 26: 441–449.
Duka T, Tasker R, Stephens DN (1998). Alcohol choice and outcome expectancies in social drinkers. Behav Pharmacol 9: 643–653.
Erblich J, Earleywine M, Erblich B (2001). Positive and negative associations with alcohol and familial risk for alcoholism. Psychol Addict Behav 15: 204–209.
Gordon M (1986). Microprocessor-based assessment of attention deficit disorders (ADD). Psychopharmacol Bull 22: 288–290.
Green AI (2005). Schizophrenia and comorbid substance use disorder: effects of antipsychotics. J Clin Psychiatry 66 (Suppl 6): 21–26.
Green AI, Burgess ES, Dawson R, Zimmet SV, Strous RD (2003). Alcohol and cannabis use in schizophrenia: effects of clozapine vs risperidone. Schizophr Res 60: 81–85.
Holdstock L, de Wit H (1998). Individual differences in the biphasic effects of ethanol. Alcohol Clin Exp Res 22: 1903–1911.
Khantzian EJ (1997). The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry 4: 231–244.
Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R (2003). NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berlin) 169: 215–233.
Krystal JH, Tabakoff B (2002). Ethanol Abuse, Dependence, and Withdrawal: Neurobiology and Clinical Implications. Lippincott Williams and Wilkins: Philadelphia.
Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS et al (1995). SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med 36: 1182–1190.
Lawson DM, Nathan PE, Lipscomb TR (1980). Guidelines for the administration of alcohol to human subjects in behavioral research. J Stud Alcohol 41: 871–881.
Madden PA, Bucholz KK, Martin NG, Heath AC (2000). Smoking and the genetic contribution to alcohol-dependence risk. Alcohol Res Health 24: 209–214.
Madden PA, Heath AC, Starmer GA, Whitfield JB, Martin NG (1995). Alcohol sensitivity and smoking history in men and women. Alcohol Clin Exp Res 19: 1111–1120.
Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM (1993). Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res 17: 140–146.
Newlin DB, Thomson JB (1990). Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull 108: 383–402.
Noordsy DL, Drake RE, Teague GB, Osher FC, Hurlbut SC, Beaudett MS et al (1991). Subjective experiences related to alcohol use among schizophrenics (comment). J Nerv Mental Dis 179: 410–414.
Overall JE, Gorham DR (1962). The Brief Psychiatric Rating Scale: a comprehensive review. Psychol Reprints 10: 799–812.
Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger Jr JI (1996). Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J Stud Alcohol 57: 368–377.
Sobell LC, Toneatto T, Sobell MB, Leo GI, Johnson L (1992). Alcohol abusers' perceptions of the accuracy of their self-reports of drinking: implications for treatment. Addict Behav 17: 507–511.
Spitzer RL, Williams JBW, Gibbon M, First MB (1990). Structured Clinical Interview for DSM-III-R-Patient Edition (SCID-P, Version 1.0). American Psychiatric Press: Washington, DC.
Acknowledgements
We acknowledge (1) the critical clinical research contributions of the Biological Studies Unit, West Haven VA Medical Center including Elizabeth O'Donell, RN; Angelina Genovese, RN; Sonah Yoo, RPh; Robert Sturwold, RPh, and (2) the statistical expertise of Rong Wang, MPH, and Brian Pittman, MS. This study was supported by the National Institute of Alcohol Abuse and Alcoholism (R03 AA11413-02 to DCD; KO5 AA014715-01, I-P50 AA-12870-04 to JK). We also acknowledge support from the (1) Department of Veterans Affairs (Schizophrenia Biological Research Center, Alcohol Research Center, National Center for PTSD and Merit Review Program (JK), (2) National Institute of Mental Health (RO1 MH61019-02 to DCD) (P50 MH44866-15 to JK), (3) National Institute of Drug Abuse 1 DA12382-01 to DCD), (4) Stanley Medical Research Institute (DCD), and (5) Donaghue Foundation (DCD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D'Souza, D., Gil, R., Madonick, S. et al. Enhanced Sensitivity to the Euphoric Effects of Alcohol in Schizophrenia. Neuropsychopharmacol 31, 2767–2775 (2006). https://doi.org/10.1038/sj.npp.1301207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301207
Keywords
This article is cited by
-
Subjective response to alcohol in young adults with bipolar disorder and recent alcohol use: a within-subject randomized placebo-controlled alcohol administration study
Psychopharmacology (2023)
-
Differenzialdiagnostische Unterscheidung zwischen substanzinduzierten und primären Psychosen:
Der Nervenarzt (2022)
-
The safety of studies with intravenous Δ9-tetrahydrocannabinol in humans, with case histories
Psychopharmacology (2012)
-
Genetic factors influencing alcohol dependence
British Journal of Pharmacology (2008)