Abstract
Although dopamine (DA) effects in the prefrontal cortex (PFC) have been studied extensively, the function of steady-state ambient levels of DA in the regulation of afferent excitatory transmission in PFC pyramidal neurons remains relatively unexplored. Using intracellular sharp-electrode and whole-cell recordings combined with intracellular labeling in brain slices, we found that D1/D5 receptor blockade did not alter synaptic responses in the PFC, but D1/D5 receptor activation consistently enhanced recurrent synaptic excitation in the majority of pyramidal neurons tested. In contrast, D4 receptor blockade resulted in an evoked complex multiple spike discharge pattern that contained both early and late (presumably multisynaptic) components of the evoked response that is contingent upon the preservation of axon collaterals of the neuron under study. Moreover, GABAergic interneurons were found to play a role in both responses; blockade of GABAa-mediated inhibition caused bath application of DA to convert monosynaptic excitatory postsynaptic potentials (EPSPs) to complex spike bursts riding on the late component of the EPSP. On the other hand, during the blockade of GABAa-mediated conductances, administration of a D4 receptor antagonist failed to facilitate evoked multiple spike discharge. Morphological analysis of axon collaterals of labeled neurons revealed that neurons in which the D4 receptor blockade induced the putative polysynaptic response had axon collaterals that were largely preserved. These data suggest that DA exerts a bidirectional modulation of PFC pyramidal neurons in brain slices provided that local network connections with interneurons are preserved, with D4 receptors under tonic stimulation by ambient low levels of DA, whereas D1/D5 receptors activated upon phasic DA input.
Similar content being viewed by others
INTRODUCTION
The major afferents to the prefrontal cortex (PFC) are glutamatergic, and dopamine (DA) receptors are known to play a major role in the control of glutamate tone in the PFC (Sesack et al, 2003; Seamans and Yang, 2004). Functional studies have revealed that the D1 receptor family is involved in working memory and other cognitive tasks (Williams and Goldman-Rakic, 1995; Goldman-Rakic and Selemon, 1997). However, physiological studies of the D2 receptor family have been hampered by the lack of a ligand selective for the various receptor subtypes, in particular the D4 receptor subtype, one of the proposed target sites of antipsychotic drugs (Arvanov and Wang, 1997; Chen and Yang, 2001). The pharmacological roles of D4 receptors have begun to be elucidated, including a role in the performance of cognitive functions involving the prefrontostriatal system in rats (Jentsch et al, 1999; Zhang et al, 2004) and monkeys (Arnsten et al, 2000), and D4 receptors may be overexpressed in schizophrenia subjects (Seeman et al, 1993; Sumiyoshi et al, 1995; but also see Lahti et al, 1996; Reynolds and Mason, 1995), in which a primary DA dysfunction is implicated (Grace, 2000; Lewis, 2000; Goldman-Rakic et al, 2004). The recent demonstration of a preferential localization of D4 receptors on parvalbumin (PV)-containing GABAergic interneurons in the PFC (Mrzljak et al, 1996; Wedzony et al, 2000) suggests a potential involvement of D4 receptors in the regulation of activity of intrinsic GABAergic neurons that may exert a powerful feedforward inhibitory influence on pyramidal neurons. Although much is known about the effects of exogenously applied DA on PFC neuron excitability (see below), the function of tonic activation of DA receptors by steady-state ambient levels of DA remains relatively unexplored in the PFC. Of particular interest in this regard are the DA D4 receptors that are closely tied to DA dysfunction in the PFC as stated above.
Most in vitro studies have consistently demonstrated a facilitatory action of D1 receptor activation on membrane excitability (Penit-Soria et al, 1987; Yang and Seamans, 1996; Shi et al, 1997; Henze et al, 2000; Wang and O'Donnell, 2001). Whole-cell recording studies have further revealed that DA activates D1 receptors to enhance NMDA-mediated responses in both PFC and striatum (Cepeda et al, 1993; Cepeda and Levine, 1998; Zheng et al, 1999; Seamans et al, 2001a; Chen et al, 2003; Tseng and O'Donnell, 2004). On the other hand, the observed inhibitory action of DA (Gulledge and Jaffe, 1998) has been attributed to activation of GABA interneurons via D1 (Gorelova et al, 2002) or D2 receptor activation (Retaux et al, 1991; Grobin and Deutch, 1998). Evidence for an inhibitory action of D2–D4 receptors in the frontal cortex is derived from studies in which D4-deficient mice exhibit spontaneous hyperexcitability in PFC pyramidal neurons (Rubinstein et al, 2001); however, this could be due to enhanced expression of NMDA and D1 receptors in D4 knockout mice (Gan et al, 2004). In contrast, a D2 subtype (D4)-mediated DA facilitatory action on membrane excitability in PFC pyramidal neurons was also reported (Ceci et al, 1999), and D4 receptor activation decreases GABAA receptor-mediated synaptic currents in dissociated prefrontal pyramidal neurons (Wang et al, 2002). Thus, there is no consensus as to which DA receptor subtype is involved in the inhibitory action of DA on PFC neuron excitability. As to the DA regulation of synaptically evoked responses in brain slices, most in vitro studies have used intracortical fiber stimulation within 100–300 μm of the soma recorded and reported different DA actions via D1 receptors in the regulation of intracortically evoked synaptic responses (Law-Tho et al, 1994; Urban et al, 2002; Seamans et al, 2001a; Gonzalez-Islas and Hablitz, 2003). Thus, a DA D1-mediated depression on both AMPA and NMDA components of excitatory postsynaptic potentials (EPSPs) (evoked by layer I or layer VI intracortical stimulation) was reported in rat prefrontal pyramidal neurons using high doses of DA (50–100 μM plus DA uptake blocker; Law-Tho et al, 1994). In contrast, whole-cell recording studies have shown that both low (0.5–7.5 μM) and high (10–50 μM) doses of D1 agonist, while slightly suppressing the AMPA component, enhance the NMDA component of intracortically evoked EPSCs in layer V pyramidal neurons (Seamans et al, 2001a) or the nonisolated EPSCs in layer II/III pyramidal neurons of the rat PFC (Gonzalez-Islas and Hablitz, 2003). It is unclear why the reported DA actions via D1 receptors in the PFC may differ in their effect on synaptic regulation.
In this study, conventional sharp-electrode intracellular and whole-cell current-clamp recordings coupled with intracellular labeling techniques were performed in frontal cortical brain slices to examine the effects of DA on excitatory synaptic responses, in particular, to determine whether the steady-state ambient DA levels might tonically activate the two DA receptor subtypes in the regulation of afferent-evoked synaptic responses. The synaptic responses were evoked by stimulating PFC afferent fibers in the corpus callosum to avoid potential artefacts due to current spread from local (intracortical) stimulation sites as used in previous studies. Low doses of DA (5–10 μM) were primarily employed in the present study to preferentially activate presynaptic receptors and high-density postsynaptic receptors, such as those located on GABAergic interneurons (Le Moine and Gaspar, 1998; Mrzljak et al, 1996). High concentrations of DA or its agonists (20–30 μM; Seamans et al, 2001a; Zheng et al, 1999) were also tested to verify that any observed lack of effect on membrane excitability or synaptic responses was not due to inadequate receptor activation by the lower (ie 10 μM) doses. These afferent-evoked excitatory synaptic responses and their modulation by DA were analyzed in terms of their mono- vs polysynaptic components (Berry and Pentreath, 1976; Chen and Yang, 2001) as well as their axonal morphology in 350 μm thick brain slices. The specific DA receptor subtype involved in the responses was examined by use of selective DA agonists and antagonists, that is, D1/D5 receptor agonists (SKF 38393 and SKF 81297), D2/D4 receptor agonists (quinpirole) or D4 receptor selective agonist (PD168077; Glase et al, 1997) and D4 receptor antagonists (PD101387 and U101958; Merchant et al, 1996, and L745870; Patel et al, 1997). Portions of this work were presented previously in abstract form (Onn and Grace, 1999).
MATERIALS AND METHODS
In Vitro Brain Slice Preparation
Six- to 8-week old developmentally mature (Zhu, 2000) male Sprague–Dawley rats (175–250 g) were used for both sharp-electrode (n=68) and whole-cell recordings (n=77). Rats were deeply anesthetized with 8% chloral hydrate (400 mg/kg, i.p.) before they were perfused transcardially with physiological saline in which sucrose (252 mM) was substituted for NaCl (252 mM sucrose, 5 mM KCl, 1.2 mM KH2PO4, 2.4 mM CaCl2, 1.3 mM MgSO4, 26 mM NaHCO3, 10 mM glucose, and saturated with 95% : 5% O2 : CO2, pH=7.23; Aghajanian and Rasmussen, 1989). Following decapitation, the brain was rapidly removed, blocked, and attached onto the chamber of a DSK vibratome (Ted Pella, USA) that was filled with ice-cold physiological saline saturated with 95% : 5% O2 : CO2. Tissue slices (350 μm in thickness) containing PFC (including anterior cingulate Cg 3 area and/or prelimbic cortices) were cut in a coronal (A-P coordinate: −2.7 to −3.7 mm anterior from bregma), sagittal (M-L: 0.4–1.2 mm lateral from midline), or horizontal plane (D-V: −3.1 to −4.1 ventral from bregma) to verify that any observed effect was not due to the plane of section of the brain slices. Tissue slices were immediately placed into incubation vials containing the oxygenated physiological saline solution at 34°C for at least 1 h before being transferred to a submersion-type chamber for recording using sharp or whole-cell electrodes (Onn et al, 2003). All rats used in this study were handled in strict accordance with the USPHS publication Guide for the Care and Use of Laboratory Animals and the specific experimental protocols were approved by the Institutional Animal Care and Use Committee of Drexel University College of Medicine and of the University of Pittsburgh.
In Vitro Intracellular Recording and Labeling Using Sharp Electrodes
Recording electrodes were constructed from 1 mm o.d. Omegadot (WPI, Sarasota, Fl) borosilicate glass tubing using a Flaming-Brown p-80/PC horizontal puller and filled with 1 M KAc containing 2% biocytin (with average electrode impedance of 75–150 MΩ as measured in situ). Intracellular recordings were performed using a NeuroData 383 Intracellular Amplifier, with current injected across a bridge circuit integral to the preamplifier. Biocytin-filled intracellular electrodes were used to obtain current-clamp recordings from PFC neurons in 350 μm thick brain slices in a submersion-type chamber maintained at 31–32°C. The electrodes were lowered into the PFC by referring to anatomical landmarks, including the anterior commissure, septum, and cortical white matter according to a rat brain stereotaxic atlas. After impalement of a cell, 0.2–0.5 nA constant hyperpolarizing current was applied to achieve a stable impalement; baseline physiological data were then collected at resting membrane potential. Only one cell per slice was recorded and injected in order to correlate cell morphology and location with the pharmacological responses.
The input resistance was calculated by measuring the membrane voltage deflections produced in response to 150 ms duration constant hyperpolarizing current pulses (0.2–1.0 nA) delivered across a bridge circuit integral to the preamplifier. Rheobase current, defined as the amplitude of current required to generate the first spike discharge, was used to assess the postsynaptic membrane excitability (Onn et al, 2003), in conjunction with the assessment of spike threshold. Action potential amplitude was measured as the difference between the spike threshold and peak of the spikes evoked by depolarizing current pulses (150 ms duration). Spike threshold was defined as the onset of the fast rising depolarizing phase of the action potentials. After obtaining drug-induced pharmacological responses, cells were injected with biocytin using 0.5–1 nA depolarizing current pulses delivered at 2–3 Hz (Onn et al, 1993, 2003; Onn and Grace, 2000). The slices with biocytin-injected cells were postfixed with 4% paraformaldehyde in 0.1 M phosphate buffer and cryoprotected in 20% sucrose solution before subjecting to histochemical staining for biocytin using avidin–biotinylate complex (Vector, CA) (Onn et al, 1993, 2003). In brief, brain slices were subjected to a freeze–thaw procedure (Onn et al, 1993) to facilitate the penetration of the ABC reagent used for staining for biocytin, followed by a 24-h incubation with avidin–biotinylated complex in 0.1 M phosphate buffer containing 0.5% Triton X-100, and a 30 min reaction with 3,3′-diaminobenzidine (DAB) (Vector Laboratories), with buffer rinsing between all steps. The slices were then cleared with 100% dimethysulfoxide (DMSO) for 20 min (Grace and Llinas, 1985) and mounted in DMSO or mounted on gelatinized slides, air-dried, dehydrated, cleared, and coverslipped with Pro-Texx (Lerner Laboratories, Pittsburgh, PA) for microscope examination using a Zeiss photomicroscope. Some brain slices were cut into 100 μm thick sections before subjected to staining for biocytin, and subsequent microscopic examination showed similar morphological details with respect to the morphology of axon collaterals as described in the results. Anatomical images were processed using Adobe Photoshop and physiological traces were plotted with Adobe Illustrator or Origin Software.
In Vitro Whole-Cell Recordings
Whole-cell patch electrodes (5–7 MΩ) were pulled from 1.5 mm glass pipettes (WPI) using a Brown and Flaming horizontal puller (model P-97; Sutter Instruments). The pipette was filled with a solution containing (in mM): 115 K-gluconate, 10 HEPES, 2 MgCl2, 20 KCl, 2 Mg-ATP, 2 Na2-ATP, 0.3 GTP, and 0.5% biocytin (pH 7.3; 295 mOsm). Whole-cell recordings were performed in a submersion-type chamber maintained at 31–32°C and the slices were superfused with oxygenated ACSF at a flow rate of ∼1 ml/min under the control of a peristaltic pump. The ACSF solutions contained (in mM): 128 NaCl, 3 KCl, 2 CaCl2, 2 MgSO4, 24 NaHCO3, 1.25 NaH2PO4, and 10 D-glucose. Cells were visualized using an Olympus BX50WI (40 × infrared lens) with infrared differential interference contrast microscopy (IR/DIC). Recordings of visualized cortical neurons were made in current-clamp (bridge) mode with an Axoclamp-2B (Axon Instruments). The output signal was low-pass-filtered at 10 KHz, amplified and digitized at 15 KHz, and acquired using pClamp/Digidata 1322A (Axon Instruments). Cells with evoked firing that showed signs of a washout effect (often their evoked firing was reduced significantly within 5 min after the initial gigaohm seal was broken) were discarded. Excluded also were cells with unstable membrane potentials or input resistances (>10% changes) before drug application.
The steady-state membrane voltage deflection in response to a 100–300 pA hyperpolarizing pulse was used to monitor the resting input resistance of a cell. Membrane excitability was measured by counting the number of action potentials evoked by depolarizing current pulses (1 s duration) over a range of currents (50–600 pA). The number of spikes (with five repetitions) was compared before and after drug application. Intracellular labeling with biocytin was carried out by allowing biocytin to diffuse from the electrode into the cell during recording. At the end of recordings, slices were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer overnight at 4°C, and transferred to 20% sucrose solution before subjecting to histochemical staining for biocytin using avidin–biotinylate complex as described above (see the section for intracellular recording and labeling). The figures were plotted with Origin software.
Afferent Fiber Stimulation and Drug Application
Afferent fibers were stimulated using a bipolar twisted-pair stimulating electrode placed in the corpus callosum (ie white matter) to evoke EPSPs and spike discharge in the recorded PFC pyramidal neurons. The distance between stimulation electrodes to the recording site in layer V/VI neurons was between 500 and 1000 μm, which is substantially greater than that commonly used for intracortical local stimulation sites (ie 70–200 μm; see Introduction). The stimulation parameters were 0.2 ms pulses of 0.01–0.5 mA, except in a few cases (using >1 mA) in neurons with apparent high spike threshold (ie no spike discharge despite evoking a full amplitude EPSP). When studying orthodromic responses, stimulation intensity was adjusted to elicit spike discharge in approximately 30–50% of the trials to minimize saturation effects. The antidromic threshold was higher than the orthdromic threshold for intracortically evoked responses in deep layer pyramidal neurons, consistent with a previous study on thalamocortical slices involving the auditory cortex (Rose and Metherate, 2001). Thus at low-stimulus intensities, intracallosal fiber stimulation reliably evoked orthodromic afferent-evoked responses in deep layer pyramidal neurons with limited antidromic activation of these output neurons.
These synaptic parameters were examined before and after bath application of DA (5–30 μM), D1 receptor agonists (SKF 38393 and SKF 81297; 10 μM, except in a few cases in which high doses of SKF 38393 (20–30 μM) were used). D2/D4 receptor agonist (quinpirole; 10 or 20 μM), D4 receptor agonist (PD 168077; 20 μM; Tocris), and D4 receptor antagonist (PD-101387 or U-101958 and L-745870; 20–50 μM; RBI) were tested at low and high doses. D1/D5 receptor antagonist (R(+) SCH-23390 HCl, 10 μM) and GABAa receptor antagonist (bicuculline, 10 μM) or picrotoxin (25 μM) were present continuously to test the DA action in the blockade of the D1 and GABAa receptor function. D(−)-2-amino-5-phosphonopentanoic acid (D-APV: 20–50 μM, Tocris) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX: 10 μM, RBI) were used to identify the AMPA- vs NMDA-mediated components of EPSPs. All drugs were purchased from Sigma, unless indicated otherwise. All drugs were first dissolved in distilled water or DMSO at high concentrations to produce stock solutions, which were stored at −20°C. Drug-containing solutions were prepared by diluting them in the oxygenated ACSF-containing reservoir bottle to achieve the working concentration; this concentration of drug required approximately 1 min to reach the chamber and 30 s to completely replace the solution in the chamber. To minimize oxidization of DA or D1/D5 receptor agonist, ascorbic acid (10 μM in final concentration) was added to the superfusate; thus, both control and drug-containing ACSF contained the antioxidant which in previous studies was found not to affect neuronal properties (Onn et al, 2003). The onset of the drug effects described in the results was calculated from the time that the perfusion lines were switched. Drug effects were first measured at 5 min of drug application (unless indicated otherwise) by evaluating the mean peak values of EPSPs from predrug baseline (of five repetitions) and from the drug washout at 10–20 min.
Statistical Analysis
Data were calculated as means±standard error of the mean (SEM). Paired Student's t-test was used for within-subject comparisons (ie comparing before and after drug application to a cell under study) and unpaired Student's t-test used for between-subject comparisons (ie between the means obtained from different neurons). A nonparametric χ2 test was used to compare the probability of spike discharge before and after drug application. Statistical significance was set at p<0.05 for all experiments.
RESULTS
Intracellular sharp electrodes and whole-cell recording electrodes, both filled with biocytin, were used in this study. A total of 177 neurons recorded in 177 brain slices (85 coronal, 52 horizontal, and 40 sagittal slices; one neuron per slice) were identified electrophysiologically as pyramidal neurons (Yang and Seamans, 1996; Onn and Grace, 2000), with 107 cells morphologically verified to be located in layer III/V border (n=44) or in layer V/VI (n=63) of the medial PFC (including the anterior cingulate cortex, Cg 3 area, and prelimbic cortex). No significant differences were noted in the observed effect of DA between neurons recorded in layer III/V border (Figure 2a3; Figure 5b) vs in layer V/VI border (Figure 2b and c; Figure 5c), and since both layers II/III and layers V/VI in the rat PFC receive DA inputs (Berger et al, 1991), the results were pooled for analysis. In 79 cells recorded with sharp electrodes and 98 cells with whole-cell electrodes, we examined the effects of DA, D1/D5 receptor agonists, and D2/D4 receptor antagonists on membrane excitability and afferent-evoked EPSPs/spike discharge. In this way, we could examine the response while controlling for washout (with sharp electrodes) and with gigaohm seal (whole-cell recordings). In no case could differences in the response be attributed to the recording methodology, except (as noted below) the tendency to record from more superficial neurons with truncated axonal processes in the visualized whole-cell recordings. The basic membrane properties of neurons recorded with whole-cell and sharp electrodes are summarized in Table 1.
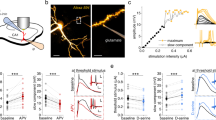
Afferent stimulation of the white matter evoked mono- and polysynaptic responses in identified PFC pyramidal neurons, with corresponding axon collaterals preserved in the brain slice under study (using whole-cell recording technique). Current-clamp recordings show constant latencies for early EPSP/spike (a), a monotonic increase in evoked EPSP amplitude with increasing stimulus strength (a1), and the ability to follow 20 Hz train stimulation (a2), consistent with a monosynaptic event, that is, Type I response pattern. Each arrow indicates the stimulus onset and artefact. Neurons exhibiting the Type I response pattern typically had little (eg restricted to basilar dendrites; a3) or moderate (eg up to middle layers; b2) preservation of their intracortical axon collaterals. On the other hand, variable onset latencies for late EPSP/spike (ie the second spikes in b1 and c1), the rapid recruitment of high-amplitude events with small increases in stimulus strength (c1), and the inability to follow high-frequency trains of impulses (see Figure 7 a2) are consistent with a polysynaptic process, that is, Type II response pattern. Neurons displaying this Type II response pattern in whole-cell recordings also exhibited extensively ramifying axon collaterals ascending from somatodendritic field to middle layers, in contrast to the superficial layer ramification pattern that is more frequently observed in the Type II neurons recovered during sharp-electrode intracellular recordings (see Figure 1 c1). This may be due to the fact that visualized whole-cell recordings tend to record from neurons located closer to the surface of slices than sharp-electrode intracellular recordings. (a1, a2, a3) show the corresponding electrophysiological and morphological characteristics typical of the Type I response neurons in whole-cell recordings (ie with limited preservation of axon collaterals). (b1, b2) show the corresponding electrophysiological and morphological characteristics typical of the Type I/II borderline neurons (ie with numerous axon collaterals confined to the somatodendritic field). (c1, c2) show the corresponding electrophysiological and morphological characteristics typical of the Type II response neurons that have axon collaterals that extend beyond middle layers. Open arrows mark axon collaterals. (a1) A Type I neuron displayed a 21 mV-sized EPSP (at 1.1 mA), and a 9 ms latency spike at –46 mV spike threshold (at 1.3 mA). (b1) A mixed Type I/II neuron displayed an all- or none response, with no response at 0.1 mA, whereas spike discharge at 0.2–0.5 mA. The first spike latency was shortened from 10 to 7 ms as increasing stimulus strength from 0.2 mA to 0.3, 0.4, and 0.5 mA, without altering the spike threshold (−48 mV). The second spike of two spikes evoked at 0.4 and 0.5 mA occurred at slightly longer latency (13.1 ms) and higher threshold (−41 mV) than the first spike. (c1) A Type II neuron displayed a 4 mV IPSP (at 0.3 mA), 10 mV-EPSP (at 0.4 mA), and one spike (at 0.5 mA) or two spikes (at 0.6 mA). The second spike of the two spikes evoked at 0.6 mA occurred at significantly longer latency (42.4 ms) and higher spike threshold (−47 mV), compared to the first spike (7 ms latency; −65 mV spike threshold). (a3, c2) DMSO-mounted horizontal slices. (b2) A protex-mounted coronal slice. Scale bars: a3, b2, and c2=40 μm.
The facilitatory action of DA during blockade of GABAA conductances on excitatory synaptic transmission in a Type I response PFC pyramidal neuron is blocked by coadministration of the D1 receptor antagonist, SCH 23390 (10 μM). (a1, a2, a3) Sharp-electrode recordings show that administration of SCH 23390 (a2) caused a nonsignificant decrease (by 1–2 mV) in EPSP amplitude, suggesting the lack of D1/D5 receptor tonic stimulation by the ambient low levels of DA. Furthermore, SCH 23390 pretreatment prevented facilitation of evoked responses normally produced by DA superfusion (a3). Asterisks in a1–3 indicate antidromic spikes that were evoked at 1 mA. No orthodromic spikes were evoked despite using high stimulus intensities (1 mA). These voltage tracings were obtained from one of the neurons (marked by numerical numbers 1 and 2) exhibiting dye coupling identified in the border of layers III/V of prelimbic cortex in a coronal slice (b1, low and b2 high magnification). Few axon collaterals (open arrows), which were only confined to basilar dendrites, were recovered in the Type I response neuron (show in a). The asterisk in (b1) indicates the location of afferent stimulation in the corpus callosum (cc), that is, 500 μm away from the soma located in the border of layers III/V (b1, b2). (c) Another incidence of coupling identified in layer V of infralimbic cortex in a coronal slice following DA application (in the presence of SCH 23390 and bicuculline); in this case, moderate axon collaterals (open arrows) were observed up to layer III in one of two coupled neurons in which a Type I/II borderline response pattern was recorded. Scale bar, b1=250 μM, b2 and c=50 μM.
None of the cortical neurons recorded in these slices were spontaneously active, and only fired spikes in response to membrane depolarization or afferent fiber stimulation. Both membrane excitability and afferent-evoked EPSPs/spike discharge were examined before and after bath application of DA (5–10 μM, low dose; 30 μM, high dose), D1/D5 receptor partial or full agonists (SKF 38393 or SKF 81297: 10 μM or 20–30 μM, high dose), and D4 receptor antagonists (PD-101387; U-101958; L-745870: 20 μM, low dose, 40–50 μM, high dose). In addition, a D2/D3/D4 receptor agonist (quinpirole; 10 or 20 μM) or a D4 receptor selective agonist (PD168077; 20 μM) was applied to confirm the involvement of the D4 receptor subtype in the DA-mediated response. D2 or D4 receptor agonists administered at the 10 μM dose had no effect on membrane excitability or synaptic responses in the pilot study, thus 20 μM was used to confirm that the lack of response (see Figure 10) was not dose-related, that is, due to insufficient receptor activation. Both high/low dose ranges used in the present study were all within the range commonly used by others, for example, D1 receptor full agonist SKF 81297 (0.5–50 μM; Seamans et al, 2001a), D1 receptor partial agonist SKF 38393 (10–100 μM; Gonzalez-Islas and Hablitz, 2003), D2/D3/D4 receptor agonist, quinpirole, or D4 receptor selective agonist PD 168077 (10–20 μM; Price and Pittman, 2001; Wang et al, 2002; Gonzalez-Islas and Hablitz, 2003), or D4 receptor antagonists (40–50 μM; Price and Pittman, 2001). Lastly, the pharmacological role of the D1/D5 and D2/D4 receptors in influencing PFC pyramidal neuron activity via modulation of GABAergic feedforward local circuits was examined in slices superfused with bicuculline (10 μM)- or picrotoxin (25–50 μM)-containing buffer.
In the presence of a GABAA chloride channel blocker (picrotoxin), D4 receptor blockade (U-101958; 40 μM) failed to facilitate the afferent-evoked early or late component responses (a2) in a deep layer pyramidal neuron with Type II response pattern during whole-cell recordings; this neuron exhibited extensive axon collaterals preserved in a horizontal slice, i.e., across all laminae to layer II. Asterisks in (a) and (b) indicate antidromic spikes that were not affected by DA (Gulledge and Sturt, 2003). Following 30 min of rinsing in a picrotoxin-free buffer (b1), subsequent bath application of a D4 agonist (PD168077; 20 μM) was able to suppress the evoked polysynaptic response (b2, 10 min). This suppression was reversed upon washout of the D4 agonist (b3, 10 min rinse). Thus, blockade of GABAA responses prevents the D4 antagonist from potentiating the polysynaptic response. This view is further substantiated by observing evoked IPSPs in pyramidal neurons by bath-applied D4 agonist or quinpirole (a D2–D4 mixed agonist). Quinpirole (20 μM; 10 min) administration did not significantly affect the response of this neuron in response to membrane depolarization, but revealed the presence of an evoked IPSP in the pyramidal neuron (d2). The decrease in evoked EPSPs (d2) may be as a result of the increase in evoked IPSPs. (e) Bar graph summarizing the group data of normalized EPSP amplitude (ie early EPSP component) indicating no significant changes by quinpirole or D4 selective agonists (in the absence of bicuculline, BIC) or by D4 antagonist in the presence of BIC. Thus, quinpirole acting like the D4 agonist did not significantly affect monosynaptic unitary EPSPs (ie early EPSP component), but attenuating late polysynaptic EPSPs and spike discharge, probably via activation of GABA interneurons (Retaux et al, 1991; Grobin and Deutch, 1998), as IPSPs were frequently evoked in the pyramidal neuron recorded during bath application of D4 agonists or quinpirole.
Monosynaptic vs Polysynaptic Responses
Two types of response patterns could be observed upon stimulation of the corpus callosum. The regular-spiking PFC output neurons mostly responded to afferent fiber stimulation with monosynaptic unitary EPSPs (avg. 13.1±4.5 mV, range from 5 to 22 mV; mean±SEM), which were blocked by the AMPA receptor antagonist CNQX (at 10 μM, not shown; Kawaguchi, 1992). Furthermore, stimulation of the corpus callosum with increasing stimulus current strength resulted in a current-dependent increase in a short-latency EPSP and spike discharge, at an average latency of 3.4±0.1 and 6.1±0.2 ms, respectively (Figure 2a). Since this component increased monotonically with increasing stimulus strength, exhibited constant latency, and could follow high-frequency (eg 20 Hz) stimulation (Figure 2a2), this was presumed to be a monosynaptically evoked potential (Berry and Pentreath, 1976; Chen and Yang, 2001), which we defined as a Type I neuron response. In contrast, a subset of neurons recorded with both techniques that were identified as layer V projection neurons (Figure 1c1; Figure 2c2) had axons that emitted extensive collaterals that ramified across all laminae, for example, ascending to superficial layers (Figure 1c1). These neurons responded to stimulation of the corpus callosum with complex evoked spikes, consisting of both early and late components that gave rise to spike discharge at an average latency of 7.6±0.2 and 28.4±0.5 ms, respectively. Since the late components did not follow high-frequency stimulation (see Figure 7a2) and did not show a smooth increase in amplitude with increasing stimulation current (Figure 2c1) and gave rise to spike discharge at variable onset latencies, these were presumed to be a combination of monosynaptic/multisynaptic origin (Berry and Pentreath, 1976; Chen and Yang, 2001). This type of neuron was classified as a Type II response pattern.
Photomicrographs taken from 350 μm thick slices illustrating biocytin-labeled Type I and Type II PFC deep layer pyramidal neurons recorded with sharp electrodes. (a) A Nissl-stained protex-mounted coronal slice. (b, c) A DMSO-mounted (b) or a protex-mounted (c) sagittal slice. The pial surface is upwards in (a), and leftwards in (b) and (c). The rostro-caudal (R-C) and dorso-ventral (D-V) plane shown is applied for both sagittal slices shown in (b) and (c). The arrow in (b) points to the rhinal fissure. Although the pyramidal somatodendritic morphology is readily discernible in the DMSO-mounted thick slice preparation (b), further examination of the same cell after mounting in protex (a, c) enhanced the resolution of the spines and axon collaterals, despite the distortion of the dendritic processes caused by tissue shrinkage due to the dehydration process (Grace and Llinas, 1985). Neurons shown in (a) and (b) exhibited a Type I response pattern (identified as described in Figure 2). (c1) A photomontage of a Type II response neuron showing the axon (marked by an arrow) arising from the soma that was traced into the white matter (WM). Before entering the white matter, each daughter axon (arrows) emitted numerous collaterals (open arrows) that ramified across all laminae up to layer II. Arrows mark the axon and its two daughter axons en passant to the nucleus accumbens (NAC). The apical dendrite (marked by the long curved arrow) was not immediately apparent (ie because of its terminating in the middle layer) in this subclass of deep layer pyramidal neurons as previously described in an in vivo preparation (Onn and Grace, 2000) and resembling the corticothalamic projection neurons in the somatosensory cortex (Zhang and Deschenes, 1997). (c2) An enlarged view of the box in c1 shows ramifying axon collaterals (open arrows) that intersected with basilar dendritic processes in deep layers. Arrowhead in (c1) indicates the location of afferent stimulation in the white matter (WM), which is marked by an asterisk in (c2). Scale bars: a=50 μm; b=100 μm; c1=50 μm; c2=5 μm.
D4 receptor blockade by bath application of PD-101389 during sharp-electrode recordings reveals a robust facilitation of intracallosal-evoked synaptic responses (ie more than a two-fold increase) in a subpopulation of pyramidal neurons in which axon collaterals were largely preserved. (a1) Low-intensity stimulation applied to afferent fibers in the corpus callosum evoked high amplitude, long duration evoked potential and spike discharge at variable latencies (15.5 or 24 ms in a1), without blockade of GABAa conductances. A small increase in the stimulus amplitude (eg from 0.05 to 0.1 mA) changed the response from an EPSP to a large amplitude, long duration depolarization with multiple spikes. (a2) These synaptic responses did not follow 4 Hz fiber stimulation, consistent with a polysynaptically mediated response. (a3) The amplitude of latter phase of the facilitation of the evoked response in the presence of D4 receptor blockade was not reversed between −50 and −70 mV. (b, c) Administration of the DA D4 receptor antagonist (50 μM; PD-101389; Merchant et al, 1996) increased the amplitude and duration of the evoked response, leading to an oscillatory type of spike discharge riding on a large depolarization (b: 5 min) and at the same spike threshold (−48 mV in a1, b, and d, and −49 mV in c), although occurring at shorter spike latency (7.2 ms) than in A1 (15.5 or 24 ms). Increasing the stimulus intensity to 1 mA (middle trace in c) from 0.1 mA (top trace in c) did not further augment the polysynaptic responses. Although this oscillatory response decreased during the application of the antagonist, the augmented response returned during the washout period (d: rinse 10 min) until a complete return to the predrug baseline at 35 min rinse (not shown). (e) Bar graph summarizing the group data of normalized EPSP amplitude altered by the D4 antagonist, thus the overall effect of blocking D4 receptors was to increase the amplitude and prolong the duration of the EPSPs (*p<0.01) in Type II pyramidal neurons by more than a two-fold increase (Table 3). Since bursts occurred so instantaneously directly from initial small EPSPs, that is, a lack of monotonic EPSP increases and therefore the initial small EPSPs cannot adequately reflect the changes induced by blockade of D4 receptors, depolarizing potentials underlying bursts were used here to reflect the changes before vs after blockade of D4 receptors.
Type I Response Neurons: D1/D5 Receptor Activation Enhanced Putative Monosynaptic Excitatory Synaptic Responses
The effects of DA acting via D1/D5 receptor stimulation were examined in a total of 93 neurons: 54 neurons were recorded with sharp electrodes (34 tested for DA and 20 for SKF compounds; with 10 cells tested for DA and five for SKF 38393 in the presence of SCH 23390) and 39 neurons were recorded using whole-cell techniques (33 tested for SKF 81297, six for DA) for confirmation of the results.
Bicuculline-free ASCF
In sharp-electrode recordings, the Type I pyramidal neurons in PFC (n=54) were characterized by the presence of a regular spike firing pattern: in response to membrane depolarization, the initial spikes were either single or doublets and were followed by an afterdepolarization (Figure 3a). In the absence of bicuculline, low doses of DA (10 μM) exerted little effect on membrane excitability, except for the reduction in the initial spike latency of current-evoked trains as reported by others (Yang and Seamans, 1996). Thus, administration of DA (10 μM) to Type I regular-spiking pyramidal neurons (n=17) did not significantly alter membrane potential (±1–2 mV), spike threshold voltage (±1–2 mV; Figure 3a), or rheobase current (1.3±0.3 vs 1.1±0.3 nA; p>0.7, n=17; Table 2, Figure 3a and e), when the number of spikes discharge during a 150 ms depolarizing pulse was evaluated over a range of currents. However, higher doses of DA (30 μM; Figure 3e) caused a significant decrease in the rheobase current (by 41±10%; 1.2±0.3 vs 0.7±0.2 nA; n=7; *p<0.05), accompanied by a depolarization of the membrane potential (avg. 3.6±1.7 mV; 1–6 mV, n=7).
Facilitatory effects of DA and D1 agonist on PFC pyramidal neurons that exhibited the Type I response pattern to intracallosal afferent stimulation. (a1, a2) Sharp-electrode recordings of current injection-evoked spikes in a layer V pyramidal neuron. DA (10 μM) did not alter the membrane potential (eg −68 mV), spike threshold voltage (eg −48 mV), or rheobase current (eg 1.3 nA; e); however, there was a reduction in the initial spike latency (eg from 60 to 35 ms; avg. 25%) during current injection-evoked spike trains as previously reported (Yang and Seamans, 1996). (b1, b2) Sharp-electrode recordings of evoked EPSPs/spikes in a layer V pyramidal neuron during stimulation of the corpus callosum (intracallosal stimulation, marked by arrows). A facilitation by DA on afferent-evoked synaptic responses was observed as a decrease in stimulus intensity required to evoke spike discharge (eg from 0.4 to 0.1 mA) without altering the amplitude of the EPSPs (f). Top trace (b1, b2): Subthreshold voltage traces of maximal EPSP amplitude before (b1: an average of five repetitions) and after (b2: an average of five repetitions) DA application (5 min). Middle trace (b1, b2): This DA facilitatory action on spike discharge was observed by decreasing the threshold stimulus strength (eg from 0.3 mA in b1 to 0.08 mA in b2). This DA facilitatory action is mimicked by D1 agonist SKF 23390 in sharp-electrode recordings (not shown) as well as in whole-cell recordings (c, d). (c, d) SKF 81297 (15 μM) caused a nonsignificant change in membrane excitability, but consistently facilitated spike discharge at the stimulus strength in four out of five trials at 0.4 mA (d2) that before D1 agonist application only produced early EPSPs (d1: 0.1–0.4 mA). The average number of spikes evoked by depolarizing current pulses at 100 pA, before vs after SKF 81292 (c1, c2), was similar (6.9±0.6 vs 7.1±0.4 spikes out of five trials; p=0.7; Student's t-test; NS). An initial estimate of cell excitability was made by examining the rheobase current that was the same for this cell (at 50 pA for before and after SKF 81292; not shown). However, D1 agonist application increased probability of spike discharge evoked by white matter stimulation at 0.4 mA, from 0% (0/5, d1: pre-D1 agonist) to 80% (4/5, d2: post-D1 agonist; p=0.0098; χ2 test). Spikes in d2 were truncated. (e, f) Bar graphs summarizing the group data of rheobase currents (e) and normalized EPSP peak amplitude (f) altered by DA and SKF 23390 during sharp-electrode recordings. Thus, in the presence of intact GABAergic events, administration of DA and D1 agonist (unless applied at high doses; e.g., 30 μM; *p<0.05) did not alter the rheobase currents or afferent-evoked EPSPs (but facilitating spike discharge by decreasing the stimulation current intensity).
This DA-induced small enhancement of membrane excitability was mimicked by application of the D1 receptor agonist: low concentrations of SKF 38393 (10–15 μM; n=10; Figure 3e) did not alter membrane excitability (1.1±0.2 vs 0.9±0.3 nA; p>0.7; NS), whereas higher doses (20–30 μM) significantly reduced rheobase currents by 34.2±11.8% (0.9±0.3 vs 0.6±0.4 nA; *p<0.05; n=10). Furthermore, in the majority of neurons recorded with monosynaptic unitary EPSPs (ie Type I response pattern), DA (10–15 μM) applied in the absence of bicuculline (Figure 3b) was found to decrease stimulation current intensities required to evoke a spike without affecting the evoked EPSP amplitude (EPSP amplitude after DA: 102.2±4.1% of predrug values; p>0.7; NS); this response was mimicked by SKF 38393 (ie 98.4±3.7% of predrug values; p>0.6; NS).
Similar DA (n=6)- or D1 (n=22)-mediated effects on the membrane excitability and afferent-evoked glutamatergic events (EPSPs and spikes) were also confirmed using whole-cell recordings from the Type I pyramidal neurons. The D1 receptor full agonist, SKF 81297 (15 μM), administered in the absence of bicuculline, did not significantly alter membrane excitability (with the rheobase current: 167±20 vs 175±21 pA; n=22; p>0.5; Figure 3c1 and c2; Table 2). Owing to the gigaohm seal in whole-cell recordings, the rheobase current was approximately one order of magnitude lower than that measured with sharp-electrode recordings (1.3±0.3 nA; see above). However, similar to intracellular sharp-electrode recordings, in the majority of whole-cell neurons that exhibited monosynaptic unitary EPSPs (ie the Type I response pattern), the D1 receptor agonist SKF 81297 (15 μM) was also observed to decrease the threshold stimulation current required to evoke spike discharge (Figure 3d1 and d2), without significantly affecting unitary EPSP amplitude (104.7± 3.4% of pre-D1 agonist values; n=22; p>0.6).
Bicuculline-containing ASCF
Following blockade of GABAa-mediated conductances by administration of bicuculline (10 μM; n=17), a more prominent DA facilitatory action was revealed; in particular, there was a significant decrease in the rheobase currents (by 35±13%; from 0.9±0.2 to 0.65±0.3 nA; *p<0.05; Figure 4a3 and d) as well as an order of magnitude lower amplitude of stimulation current required for afferent fiber stimulation to evoke single-spike discharge (eg from 0.7 to 0.09 mA; Figure 4b1 and 2). This DA facilitatory action on fast synaptic potentials in PFC pyramidal neurons was observed as a prolongation of the time to the EPSP peak (from 12.4±2.5 ms to 33.2±3.5 ms; n=17) and an increase in its peak amplitude (by 57.1±18%; 13.1±4.5 vs 22.2±4.2 mV; *p<0.05; Figure 4b2, 3, c2, 3 and d; Table 2). This facilitatory action by DA or D1 receptor agonist on the late EPSP component in PFC pyramidal neurons was not present when DA was applied to slices pretreated with SCH 23390 (n=7; Figure 4d and Figure 5) or during coperfusion of DA with the D1 receptor antagonist SCH 23390 (10 μM; n=3). Thus, in the presence of SCH 23390, there were no changes by DA in the EPSP onset latency (3.4±0.1 vs 3.4±0.1 ms), the EPSP peak latency (10.5±1.2 vs 11.1±2.7 ms), and the EPSP peak amplitude (14.3±2.7 vs 13.2±2.3 mV; Figure 4d; Table 2). SCH 23390, either alone (n=7) or in the presence of added DA (n=10; Table 2), did not significantly alter membrane excitability or synaptic responses. These data suggested that D1/D5 receptors are not tonically stimulated by the low ambient DA concentrations in the brain slice. Similar results were also obtained using whole-cell recordings in a total of 11 neurons in the presence of bicuculline (Table 2).
Facilitatory effects of DA during blockade of GABAA conductances observed with sharp-electrode recordings from PFC pyramidal neurons that exhibited the Type I response pattern to intracallosal afferent stimulation. (a1, a2) Low doses of DA significantly increase membrane excitability as shown by a leftward shift in the current-evoked response curve (a3) before and after DA application, by plotting the number of spikes discharge vs the amplitude of current injected intracellularly. An increase or decrease in the membrane excitability was defined over a range of currents applied. (b1, b2) Following bath application of DA, there was a significant decrease in the stimulus intensity required to evoke spike discharge as well as an increase in the late-phase EPSP amplitude without altering the amplitude of early EPSP (see b3, EPSP overlays before and after DA application). Although application of bicuculline (BIC) alone for 5 min did not significantly alter membrane excitability or synaptic responses, adding DA to BIC-containing buffer caused intracallosal stimulation to trigger bursts of spikes (b2, bottom trace) over a similar range of stimulation intensities (0.1–0.8 mA) as before DA application (b1: 0.1–0.7 mA). (b4) Overlay of spikes evoked before (by 0.7 mA) and after (by 0.09 mA) DA application. (c1, c2) Simultaneous application of DA and BIC (c2) to a layer III pyramidal neuron also led to burst-firing spike discharge upon afferent fiber stimulation at 1 mA (top trace) or at 6 mA (middle trace). The use of high stimulus intensity (6 mA) did not elicit spike discharge that was only evoked upon DA application in the presence of BIC (c2). Thus, independent of low and high stimulus threshold intensities characteristic of pyramidal neurons, similar enhancement on the late phase of the EPSP was induced by the DA application in the presence of BIC, either applied prior to DA (b) or together with DA (c), and this DA-enhancing effect was washed out at 15 min rinse (c4: an average of 5 repetitions). (d) Bar graph summarizing the group data of normalized rheobase currents (left) and EPSP peak amplitude (right) altered by DA and SCH 23390, in the presence of BIC (see Table 2). Thus, when GABAA-mediated transmission was blocked, DA significantly decreased rheobase current (ie enhancing membrane excitability; *p<0.05) and increased the amplitude of EPSPs (*p<0.05) that was blocked by coadministration of SCH 23390 (see Figure 5).
The Type I regular-spiking PFC output neurons that responded to DA application with an increase in membrane excitability and synaptic-evoked responses exhibited common morphological features, that is, having apical dendrites that reach the pial surface and recurrent axon collaterals confined to their basilar dendritic tree (Figure 1a and b, Figure 5b and c). Instances in which multiple neurons were labeled after a single intracellular injection (ie dye/trace coupling; Onn and Grace, 1999, 2000) were observed in four out of 10 neurons recovered and recorded with sharp electrodes in slices after exposed to SCH 23390 in bicuculline buffer (Figure 5b and c). Owing to the use of multiple drug applications during the course of the experiment, the data on drug-induced coupling cannot be properly evaluated.
Type I Response Neurons: Blockade of D4 Receptors did not Significantly Modify Monosynaptic Spike Discharge
Sixteen Type I response neurons recorded using sharp electrodes were tested with the D4 receptor antagonist PD-101367 and 22 Type I response neurons recorded with whole-cell electrodes were tested with the D4 receptor antagonist U-101958 (the same D4 receptor antagonist to PD-101367; Merchant et al, 1996).
Application of the D4 receptor antagonist PD-101387 to 16 regular-spiking ‘Type I’ pyramidal neurons that had little preservation of axon collaterals in the slices (ie did not extend beyond the deep layer where the soma was located, see Figure 1a and b, Figure 2a, Figure 5b) did not consistently alter the evoked EPSPs, but facilitated spike discharge (not shown, see Figure 9b for whole-cell recordings). Furthermore, subsequent administration of DA (20 μM, in the presence of D4 receptor antagonist) resulted only in a small nonsignificant enhancement in EPSP amplitude (+11, 19, and 22%, respectively; avg. 17±7%), as typically observed in Type I pyramidal neurons (after bath-applied D1/D5 receptor agonist in the absence of bicuculline). Therefore, in neurons exhibiting the Type I response pattern during sharp-electrode recordings, blockade of D4 receptors did not appear to produce any synaptic changes by itself except for a nonsignificant increase in evoked short-latency EPSP amplitude.
D4 antagonist administration produces little enhancement of both membrane excitability and short latency-evoked responses in Type I pyramidal neurons in which relatively few axon collaterals were preserved in slices (Figure 2 a3; b2). Both pyramidal neurons recorded with visualized whole-cell recordings exhibited the characteristic evoked spike pairs upon membrane depolarization (a1). (a and b) D4 antagonist (U-101958; 40 μM; 10 min) did not significantly alter the response to membrane depolarization (a1, 2), but facilitated the evoked EPSP in response to afferent activation (b1, 2) in a layer III pyramidal neuron (shown in Figure 2 a3) identified with Type I response pattern (b1, inset). Inset in b1 shows a graded response which is consistent with a monosynaptic event. (c1, c2, c3) Similarly, D4 antagonist U-101958 did not affect the response to membrane depolarization (not shown) or on the fast evoked EPSP (c1: 8 mV; c2: 7 mV) in a layer V pyramidal neuron (shown in Figure 2b2), but a late evoked IPSP (4.7±2.1 mV in amplitude) was revealed following rinse of the D4 antagonist (c3). Inset in c1 shows a graded response which is consistent with a monosynaptic event, i.e., Type I response pattern. (d) Bar graph summarizing the group data of normalized EPSP amplitude during whole-cell recordings in response to D4 antagonists with little enhancement in Type I pyramidal neurons.
In 22 pyramidal neurons recorded with whole-cell electrodes that exhibited the Type I synaptic response pattern (Figure 9b1-inset), blockade of D4 receptors facilitated evoked monosynaptic spike discharge without producing the late onset EPSP/spike discharge in 11 cells (Figure 9b and d). In seven of the remaining 11 nonresponding cells, upon washout of the D4 receptor antagonist prominent evoked IPSPs were revealed (avg. 4.1±1.6 mV; Figure 9c3). Both responses (ie promoting monosynaptic spike discharge and evoked IPSPs upon washout) were recorded from neurons later revealed in slices with axon collaterals largely confined to the somatodendritic field (Figure 2b2), that is, with the collateralization considerably less than those neurons that responded with complex spike bursts to the blockade of D4 receptors (Figure 8; Figure 2b). This indirect evidence suggests that administration of the D4 antagonist may induce synaptic facilitation via attenuation of afferent-evoked IPSPs.
D4 antagonist administration causes a moderate facilitation of the afferent-evoked response (ie less than a two-fold increase) observed with visualized whole-cell recordings in an identified layer V pyramidal neuron (shown in Figure 2 c2) in which a moderate preservation of axon collaterals was identified. This neuron, recorded with whole-cell technique in a horizontal slice, responded with a burst of spikes upon membrane depolarization and exhibited a Type II synaptic response pattern (ie both short-latency and long-latency EPSP components and spike discharge at variable latencies from 9 to 32 ms). This deep layer pyramidal neuron had a resting membrane potential of −62 mV, with spike overshoot >+30 mV and spike threshold voltage of −48 mV; the apical dendrite extended into superficial layers and the axon was observed to enter the white matter. Incremental increases in the amplitude of afferent stimulation (stimulus intensities from 0.1 to 1 mA (inset in a1)) revealed sudden onset of multiple components of the response rather than a graded response, suggesting a multisynaptic process. (a1–4) Multiple spike discharges elicited at 0.5 mA stimulation intensity were moderately potentiated by bath application of the D4 antagonist U-101958 (40 μM; Merchant et al, 1996) at 3 min (a2), 5 min (a3), and 10 min (a4). The D4 antagonist-facilitated response evoked by 0.5 mA threshold stimulation was not present when the stimulation strength was increased above the amplitude required to evoke a spike discharge (0.7 mA) when applied alternately with 0.5 mA stimulation (b2: 3 min; b3: 10 min; b4: rinse). Spikes were truncated for a graphic purpose. (c) Bar graph summarizing the group data of normalized EPSP amplitude during whole-cell recordings in response to D4 antagonists in Type II pyramidal neurons with a moderate facilitation (less than a 1.5-fold increase; *p<0.05; Table 3).
Type II Response Neurons: Blockade of D4 Receptors Facilitated Presumed Polysynaptic Excitatory Responses
Nine Type II response pattern neurons were recorded using sharp electrodes and tested with the D4 receptor antagonist: PD-101387 and 24 Type II neurons were recorded with whole-cell electrodes and tested with U-101958 (n=10) or L-745870 (n=14). In all cases, the results obtained with PD-101387 or U-101958 (the same D4 receptor antagonist, Merchant et al, 1996) or L-745870 (Patel et al, 1997) were similar in terms of measures of synaptic excitability (ie the responses were enhanced).
Bicuculline-free ASCF
In a subpopulation of pyramidal neurons (n=9 using sharp electrodes; Figure 1c1 and c2), low stimulus intensities (typically <0.05 mA) applied to PFC afferent in the corpus callosum were found to evoke a large amplitude, long duration depolarization, and a burst of spikes (in the absence of bicuculline), that is, a Type II response pattern (Figures 6 and 7; Table 3). These afferent-evoked repetitive spike discharges in identified Type II neurons were suppressed by low doses of DA (5–10 μM) to reveal short-latency EPSPs (n=3; Figure 6c). These pyramidal neurons with the Type II response pattern were classified as burst-firing neurons as shown by their current–voltage responses to depolarizing current pulses applied intracellularly (Figure 6a and b), and DA was found to produce a significant decrease in their membrane excitability (by 41±12%; *p<0.05; n=3; Figure 6b). Decreases in postsynaptic membrane excitability and afferent stimulation-evoked responses in these Type II neurons occurred with little effect on resting membrane potential (1–2 mV; Figure 6) and input resistance (by 5–10 MΩ).
Inhibitory effects of DA on membrane excitability and afferent-evoked synaptic responses observed with sharp-electrode recordings in a deep layer pyramidal neurons characterized by burst-firing spike discharge in response to intracallosal stimulation (the Type II response pattern). (a1, a2, a3, a4, a5) Membrane responses prior to DA application at different amplitudes of intracellular current injection. (b1, b2, b3, b4, b5) Membrane responses to current injected at rheobase amplitude in the presence of 5 μM DA (b2: 5 min), 10 μM DA (b3: 5 min), and in the rinse (b4: 10 min). (b5) DA application resulted in a rightward shift in the current–response relationship, demonstrating a decrease in membrane excitability (open circle: 5 μM DA; triangle: 10 μM DA) that returned to pre-DA conditions in rinse (diamond: 10 min rinse). (c1, c2, c3) Bath application of DA attenuated the large amplitude, long duration evoked response to intracallosal stimulation. DA was found to prolong spike latency from 4.5 ms (in c1), 9.7 ms (in c2) to 17 ms (in c3), as its concentration was increased from 0, 5, to 10 μM. The spike threshold was identified at −42, −41, and −39 mV, respectively. At higher doses of DA (c3; 10 μM), the EPSP duration was shortened to a response similar to that evoked in Type I pyramidal neurons (ie short-latency EPSPs revealed after attenuation of long-latency EPSPs). These tracings were taken from the neuron shown in Figure 1c, which is characterized by extensive axon collaterals ramifying across all laminae (up to layer II) within a 350 μm slice. Thus, DA (10 μM) significantly increased rheobase currents by 41±12% (*p<0.05) and attenuated repetitive spike discharge to reveal short-latency EPSPs in three Type II neurons examined (see Table 3).
To test the potential influences of D4 receptors on the inhibitory effects of DA on spike discharge, we first examined the effects of the D4 receptor selective antagonist PD101387 on the Type II response evoked by afferent fiber stimulation in six deep layer pyramidal neurons recorded intracellularly using sharp electrodes. In four out of the six neurons that responded with the Type II response pattern, the D4 receptor antagonist was found to prolong the evoked response, resulting in a repetitive spike discharge (Figure 7b; Table 3). Thus, bath application of the D4 receptor antagonist potentiated the late EPSP component (to 230±25% of their predrug values; p<0.01; n=4), resulting in complex spike bursts, without significant changes in the input resistance (12±4.1% of control values). There was a considerable rightward shift in the peak of the EPSP (by 97±51% of control values, Table 3), giving rise to complex spike bursts. These synaptic responses occurred with variable spike latencies (range: 7–24 ms; Figure 7a1), and did not follow 4 Hz afferent fiber stimulation (Figure 7a2), suggesting that they were likely polysynaptically mediated responses (see Figure 2c1). The late EPSP component in the afferent-evoked synaptic potential was not reversed upon hyperpolarization or depolarization of the cell membrane at potentials corresponding with the chloride reversal potential, as would be expected for responses mediated by GABAa-dependent chloride conductances (Figure 7a3). This synaptically evoked repetitive spike discharge observed during bath application of the D4 antagonist could not be replicated simply by increasing the amplitude of afferent fiber stimulation (eg from 0.1 to 1 mA; Figure 7c). The remaining two Type I/II borderline response neurons responded to the D4 receptor antagonist only with an enhancement of spike discharge during the early EPSP component (as observed in most whole-cell recordings; see next section), and were found to contain less well-preserved axon collaterals (ie the axon could only be traced up to middle layers) in slices than those four cells responding to the D4 receptor blockade with repetitive spike discharge (ie with axon collaterals that extended to the superficial layers). It should be noted that the D4 receptor antagonist effect occurred in the absence of added DA, and therefore was due to blockade of the action by the low endogenous DA levels present in the slice preparation, suggesting tonic activation of D4 receptors present in brain slices.
The extent of repetitive spike discharge observed using whole-cell recordings in 14 Type II neurons (Figure 8) was substantially less when compared to the extent of repetitive spike discharge that was observed in those Type II cells recorded with intracellular sharp-electrode recordings (Figures 6 and 7). Furthermore, this synaptic facilitation produced by blockade of D4 receptors was observed to be stimulus intensity dependent (in three cells tested with a range of stimulus intensities); that is, only occurring when the neurons were activated by a given optimal stimulus intensity. Thus, use of a lower or higher stimulus intensity than the intensity determined to be optimal (Figure 8a) for eliciting facilitation of evoked discharge by the D4 receptor antagonist did not allow the D4 receptor antagonist to elicit similar synaptic facilitation as was evoked by the optimal stimulus intensity (Figure 8b).
Effects of D2/D4 agonists in the presence or absence of GABAa-mediated conductances
In another seven Type II pyramidal neurons during visualized whole-cell recordings, we further tested the hypothesis that a GABAa-mediated inhibitory process underlies the ability of the D4 receptor antagonist to modulate the evoked response. This was done by examining the effects of the D4 receptor antagonist in the presence of bicuculline (10 μM; n=3) or picrotoxin (25 μM; n=4). Under these conditions (ie during blockade of GABAA-mediated conductances; n=7), the D4 receptor antagonist was no longer capable of facilitating the evoked response in Type II pyramidal neurons, with the peak amplitude and latency of e-EPSPs: 115±13 or 121±19% of the predrug values (p>0.7; Figure 10a1–3; Table 3). Furthermore, subsequent addition of the D4 receptor agonist PD 168077 (20 μM; Glase et al, 1997) to the bath free of bicuculline or picrotoxin (ie after complete washout of D4 antagonist and bicuculline) was found to suppress the evoked late component responses in four out of four cells tested (Figure 10b2). In general, the D4 receptor agonist did not significantly alter monosynaptic unitary EPSPs (average 98.2±3.2% of their predrug values; p>0.5), but suppressed late EPSPs (Figure 10b2). This suggests that the absence of D4 antagonist-induced facilitation in bicuculline- or picrotoxin-containing buffer (Figure 10a1–3) was due to a compromise of the GABAA-mediated inhibitory circuits by bicuculline or picrotoxin. This was further tested in three Type II pyramidal neurons by examining the effect of quinpirole, a mixed D2–D4 agonist, in bicuculline-free buffer (Figure 10c and d). In these neurons, evoked IPSPs (4.7±2.1 mV in amplitude at 22±3 ms for the onset latency) were readily uncovered in bicuculline-free buffer during application of quinpirole (Figure 10d). Similar to D4 agonist, quinpirole did not alter monosynaptic unitary EPSPs (an average of 96.8±4.1% of the predrug values; Figure 10b).
DISCUSSION
Using conventional intracellular and whole-cell recordings and labeling in cortical slices, we documented two modes of DA modulation of excitatory, presumably glutamatergic, events in identified pyramidal neurons following afferent fiber stimulation in the corpus callosum. The two modes of DA modulation appear to correspond with the Type I and Type II pyramidal neurons defined by their different synaptic responses to afferent fiber stimulation. First, D1/D5-like DA receptor activation, without consistently altering the early component of the EPSPs, consistently enhanced late EPSP-mediated spike discharge in a majority of pyramidal neurons (ie Type I response neurons) recorded when GABAA-mediated inhibitory transmission was blocked. Second, contingent upon GABA inhibitory circuits, D4 receptor blockade by D4 selective antagonists resulted in an evoked complex multiple spike discharge pattern in the Type II deep layer pyramidal neurons, in which the axon collaterals are largely preserved in the slice under study. Furthermore, D2/D4 receptor activation, without consistently altering the early component of monosynaptic EPSPs, suppressed the late, presumably polysynaptic, responses characteristic of recurrent excitatory synapses. Thus, DA facilitates excitatory network interactions via D1/D5 receptor activation, whereas D2/D4 receptor activation suppresses late excitatory drive without affecting the early excitatory response. Blockade of D4 receptors (but not D1/D5 receptors) enhances the late component glutamatergic events leading to complex spike bursts, suggesting that D4 receptors under tonic stimulation by the low ambient DA concentrations in the brain slice but D1/D5 receptors activated only upon phasic DA input.
We demonstrate that bath application of DA acting via D1/D5 receptors exerts a facilitatory action on intracallosal afferent-evoked excitatory synaptic responses in most Type I pyramidal neurons. This synaptic facilitation by intracallosal fiber stimulation was observed as increases in the late EPSP amplitude and duration, decreases in the stimulus intensity needed to evoke spikes, and during blockade of GABAA conductances, a switch to burst-firing spike discharge arising from the late component EPSPs. Thus, blockade of GABAa-mediated inhibition by low doses of bicuculline enhanced the facilitation; therefore coactivation of GABAergic circuits may be involved in partially compensating for the DA facilitation of late EPSP-mediated events. This is consistent with results showing that D1 receptor activation increases the GABA interneuron excitability (Seamans et al, 2001b). This facilitatory effect of DA, in the presence of bicuculline, was blocked by coadministration of SCH 23390 (10 μM), suggesting the involvement of D1/D5 receptors. This is consistent with studies showing that DA acts on D1/D5 receptors to enhance intracortically evoked EPSCs in layer II/III (Gonzalez-Islas and Hablitz, 2003) and layer V pyramidal neurons of rat PFC (Seamans et al, 2001a), without affecting mini-EPSCs (Zhou and Hablitz, 1999), that is, a direct postsynaptic action on the pyramidal neuron. In contrast, studies using paired-neuron stimulation/recordings suggest that D1 receptors may produce an inhibitory response via attenuation of excitatory transmission in the ferret (Gao et al, 2001) and in the primate (with coactivation of D2 receptors, Urban et al, 2002). Although our data do not directly address pre- vs postsynaptic events, the D1 enhancement of the late component of the evoked EPSP (ie altering the EPSP time course) is consistent with a postsynaptic interaction of D1 and NMDA receptors (Zheng et al, 1999; Seamans et al, 2001a; Chen et al, 2003; Tseng and O'Donnell, 2004). Consistently, anatomical evidence shows that D1 receptor immunoreactivity is frequently associated postsynaptically with dendritic spines and shafts that are in close proximity to asymmetrical synapses characteristic of glutamate receptors (Simley et al, 1994; Goldman-Rakic et al, 2004).
In the Type II deep layer pyramidal neurons, we also observed a DA-mediated depression of afferent-evoked synaptic responses in slices that was attributed to activation of D2/D4 receptors. The DA-mediated suppression was most evident on the late component EPSPs and spike discharge, that is, attenuating the large amplitude, long duration evoked response to afferent stimulation in the white matter (see Figure 6c2 and 3). Such a response may not be evident in many studies given its dependence on the use of low doses of DA, the presence of GABAA-intrinsic circuits, and most importantly preservation of axon collaterals in the brain slices under study. The role of GABA interneurons in affecting the DA (via D4 receptor) inhibitory effect or in affecting the D4 receptor blockade-induced repetitive spike discharge is supported by the following observations: (1) the absence of D4 antagonist-induced increase in response to afferent stimulation in slices superfused with buffer containing bicuculline or picrotoxin (Figure 10a) and (2) activation of D2/D4 receptors by the D2/D4 receptor agonist (quinpirole) or upon washout of the D4 receptor antagonist consistently uncovered afferent-evoked IPSPs in identified pyramidal neurons (Figures 9 and 10b). Furthermore, the potentiation of previously subthreshold IPSPs by D2/D4 receptor activation (see Figures 9 and 10d) is consistent with a DA enhancement of GABA release (Retaux et al, 1991; Grobin and Deutch, 1998), although activation of D2/D4 receptors was recently reported to briefly decrease GABA release probability (Seamans et al, 2001b). However, the presence of a tonic inhibitory role of D2/D4 receptors in the prefrontal cortex reported here is consistent with the observed hyperexcitability present in pyramidal neurons of D4-deficient mice (Rubinstein et al, 2001). A GABAA-mediated input at the level of dendrites of pyramidal neurons that enhances NMDA components to afferent fiber stimulation in the piriform cortex (Kanter et al, 1996) may be involved in the tonic D4 receptor-mediated regulation observed here. Nevertheless, a postsynaptic activation of D4 receptors on GABA interneurons (Mrzljak et al, 1996; Le Moine and Gaspar, 1998; Wedzony et al, 2000) that synapse onto the pyramidal neuron under study is likely to account for at least part of the DA inhibitory action observed here. Our present studies further demonstrate that D4 receptor blockade-mediated hyperexcitability is sensitive to bicuculline, and therefore likely involves GABA inhibitory circuits. This is consistent with a model whereby D4 receptor activation is necessary to allow GABAergic local circuit neurons to limit the spread of activity among interconnected pyramidal neurons (Chagnac-Amitai and Connors, 1989). The intracortical network circuitry activated by stimulation of afferent fibers in the corpus callosum (present study) is critical for observing the robust enhancement during blockade of D4 receptors on the late EPSPs to lead to complex spike bursts. It is worth noting that in every case (n=9) in which the pyramidal neurons exhibited the Type II late-EPSP component response pattern, the deep layer neurons also had largely preserved axon collaterals (ie extending to superficial layers) within the slices. We therefore propose that large numbers of GABA neurons as well as pyramidal neurons are activated by axon collaterals, in conjunction with orthodromic activation of the recorded pyramidal neuron by stimulation of afferent fibers in the corpus callosum (the white matter). Intact collaterals activate both GABA neurons and neighboring pyramidal neurons that contact the neuron under study.
The fact that many of the axonal connections are likely to be severed in 350 μm thick brain slices may explain the scarcity of neurons encountered with long-duration bursting spike discharge evoked by afferent stimulation in this preparation using adult mature rat brain slices. The late EPSPs reported here by afferent stimulation in slices taken from developmentally mature rats (ie 6–8 weeks; Zhu, 2000) resemble the late EPSPs evoked in layer II/III frontal cortical pyramidal neurons by intracortical stimulation in slices from young prepubertal rats (<6 weeks; 120–150 g; Sutor and Hablitz, 1989) or in guinea-pig neocortical neurons that were characterized as stimulus intensity sensitive (Connors et al, 1982), as similarly reported here (see Figure 8). These neurons, which were mostly recovered in cortical deep layer V following intracellular recording/labeling, had axons which ramified extensively and ascended to superficial layers, in addition to giving off several secondary axons that target subcortical structures (see Figure 1c). The morphology of this cell class resembles the morphology of corticothalamic pyramidal neurons labeled juxtacellularly in the somatosensory cortex in vivo (Zhang and Deschenes, 1997) or in vitro (McCormick et al, 1985; Chagnac-Amitai et al, 1990) that were described to have a short apical dendrite terminating in middle layers and extensive intracortical axonal collaterals that extend from deep layers to the superficial layers. The presence of recurrent axon collaterals in this cell class has long been speculated to underlie the tendency to fire in synchronous bursts (Silva et al, 1991). Thus, this population is likely under-represented as a result of the slicing procedure in young adult rat brains as used in the present study. Whether the Type I and Type II neurons represent two distinct classes of neurons, or instead reflect degree of preservation of synaptic networks, remains to be determined.
The present study also shows that the D4 antagonist was highly effective in augmenting the amplitude of late EPSPs in slices with well-preserved axon collaterals, whereas D2 or D4 receptor stimulation was weak in attenuating EPSPs. The ability of the D4 receptor antagonists to increase EPSP amplitude without the addition of exogenous agonist suggests the presence of tonic D4 receptor activation by low concentrations of ambient DA levels. DA D2/D4 receptors and mRNAs are reported to be abnormally elevated in frontal cortex of schizophrenia patients (Tallerico et al, 2001, also see Introduction) and the atypical antipsychotic drug, clozapine, has a high affinity for blockade of D4 receptors (Van Tol et al, 1991). Similar polysynaptic activity was reported following acute application of clozapine to prefrontal cortical pyramidal neurons (Chen and Yang, 2001), which may be due to enhanced release of DA and glutamate (Daly and Moghaddam, 1993) thereby leading to a D1 potentiation of NMDA receptor functions (see Introduction) and the late NMDA-mediated polysynaptic responses as observed here by D4 receptor blockade. D4 receptor proteins present in high levels in cortical layer II/III or V/VI are associated with both calbindin- and parvalbumin-containing GABA interneurons (Mezljak et al, 1996; Le Moine and Gaspar, 1998; Wedzony et al, 2000). Alterations in GABA transmission in these subclasses of GABAergic neurons have been reported in the PFC of schizophrenia brains examined post mortem (Bird et al, 1977; Akbarian et al, 1995; Lewis, 2000) and in a rat model of amphetamine sensitization (Mohila and Onn, 2005). Although D4 antagonists have not yet been reported to have antipsychotic efficacy (Lahti et al, 1998), both D4 receptor anatgonists, PNU-101387 and L-745870, were reported to prevent or improve cognitive deficits in monkeys (Arnsten et al, 2000) and in rats (Zhang et al, 2004). Thus, blockade of D4 receptors on GABAergic neurons that regulate intracortical circuits could act to diminish a hypofrontal state, if the negative symptoms (eg emotional and cognitive deficits) of schizophrenia are indeed associated with a hypofrontal state (Grace, 2000).
References
Aghajanian GK, Rasmussen K (1989). Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse 3: 331–338.
Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney Jr WE et al (1995). GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cerebral Cortex 5: 550–560.
Arnsten AF, Murphy B, Merchant K (2000). The selective dopamine D4 receptor antagonist, PNU-101387, prevents stress-induced cognitive deficits in monkeys. Neuropsychopharmacology 23: 405–410.
Arvanov VL, Wang RY (1997). Clozapine and haloperidol modulate N-methyl-D-aspartate receptor-mediated neurotransmission in rat prefrontal cortical neurons in vitro. J Pharmacol Exp Ther 283: 226–234.
Berger B, Gaspar P, Verney C (1991). Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci 14: 21–27.
Berry MS, Pentreath VW (1976). Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res 105: 1–20.
Bird ED, Spokes EG, Barnes J, MacKay AV, Iversen LL, Shepherd M (1977). Increased brain dopamine and reduced glutamic acid decarboxylase and choline acetyl transferase activity in schizophrenia and related psychoses. Lancet 2: 1157–1158.
Ceci A, Brambilla A, Duranti P, Grauert M, Grippa N, Borsini F (1999). Effect of antipsychotic drugs and selective dopaminergic antagonists on dopamine-induced facilitatory activity in prelimbic cortical pyramidal neurons. An in vitro study. Neuroscience 93: 107–115.
Cepeda C, Buchwald NA, Levine MS (1993). Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA 90: 9576–9580.
Cepeda C, Levine MS (1998). Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Dev Neurosci 20: 1–18.
Chagnac-Amitai Y, Connors BW (1989). Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition. J Neurophyiol 61: 747–758.
Chagnac-Amitai Y, Luhmann HJ, Prince DA (1990). Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J Comp Neurol 296: 598–613.
Chen G, Greengard P, Yan Z (2003). Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex. Proc Natl Acad Sci USA 101: 2596–2600.
Chen L, Yang CR (2001). Interaction of dopamine D1 and NMDA receptors mediates acute clozapine potentiation of glutamate EPSPs in rat prefrontal cortex. J Neurophysiol 87: 2324–2336.
Connors BW, Gutnick MJ, Prince DA (1982). Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol 48: 1302–1320.
Daly DA, Moghaddam B (1993). Action of clozapine and haloperidol on the extracellular levels of excitatory amino acids in the prefrontal cortex and striatum of conscious rats. Neurosci Lett 152: 61–64.
Gan L, Falzone TL, Zhang K, Rubinstein M, Baldessarini RJ (2004). Enhanced expression of dopamine D1 and glutamate NMDA recaptors in dopamine D4 receptor knockout mice. J Mol Neurosci 22: 167–178.
Gao WJ, Krimer LS, Goldman-Rakic PS (2001). Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA 98: 295–300.
Glase SA, Akunne HC, Georgic LM, Heffner TG, Mackenzie RG, Manley PJ et al (1997). Substituted [(4-phenylpipera-zinyl)-methyl]benzamides: selective dopamine D4 agonists. J Med Chem 40: 1771–1772.
Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Willaims GV (2004). Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacolgoy 174: 3–16.
Goldman-Rakic PS, Selemon LD (1997). Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 23: 437–458.
Gonzalez-Islas C, Hablitz JJ (2003). Dopamine enhances EPSCs in layer II–III pyramidal neurons in rat prefrontal cortex. J Neurosci 23: 867–875.
Gorelova N, Seamans JK, Yang CR (2002). Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol 88: 3150–3166.
Grace AA (2000). Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev 31: 330–341.
Grace AA, Llinas R (1985). Dehydration-induced morphological artifacts in intracellularly stained neurons: circumvention using rapid DMSO clearing. Neuroscience 10: 333–348.
Grobin AC, Deutch AY (1998). Dopaminergic regulation of extracellular gamma-aminobutyric acid levels in the prefrontal cortex of the rat. J Pharmacol Exp Ther 285: 350–357.
Gulledge AT, Jaffe DB (1998). Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci 18: 9139–9151.
Gulledge AT, Stuart GJ (2003). Action potential initiation and propagation in layer 5 pyramidal neurons of the rat prefrontal cortex: absence of dopamine modulation. J Neurosci 23: 11363–11372.
Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G (2000). Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol 84: 2799–2809.
Jentsch JD, Taylor JR, Redmond Jr DE, Elsworth JD, Youngren KD, Roth RH (1999). Dopamine D4 receptor antagonist reversal of subchronic phencyclidine-induced object retrieval/detour deficits in monkeys. Psychopharmacology (Berl) 142: 78–84.
Kanter ED, Kapur A, Haberly LB (1996). A dendritic GABAa-mediated IPSP regulates facilitation of NMDA-mediated responses to burst stimulation of afferent fibers in piriform cortex. J Neurosci 16: 307–312.
Kawaguchi Y (1992). Receptor subtypes involved in callosally-induced postsynaptic potentials in rat frontal agranular cortex in vitro. Exp Brain Res 88: 33–40.
Lahti RA, Roberts RC, Cochrane EV, Primus RJ, Gallager DW, Conley RR et al (1998). Direct determination of dopamine D4 receptors in normal and schizophrenic postmortem brain tissue: a[H]NGD-94-1study. Mol Psychiatry 3: 528–533.
Lahti RA, Roberts RC, Conley RR, Cochrane EV, Mutin A, Tamminga CA (1996). D2-type dopamine receptors in postmortem human brain sections from normal and schizophrenic subjects. NeuroReport 7: 1945–1948.
Law-Tho D, Hirsch JC, Crepel F (1994). Dopamine modulation of synaptic transmission in rat prefrontal cortex: an in vitro electrophysiological study. Neurosci Res 21: 151–160.
Le Moine C, Gaspar P (1998). Subpopulations of cortical GABAergic interneurons differ by their expression of D1 and D2 dopamine receptor subtypes. Mol Brain Res 58: 231–236.
Lewis DA (2000). GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Res Brain Res Rev 31: 270–276.
McCormick DA, Connors BW, Lighthall JW, Prince DA (1985). Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–804.
Merchant KM, Gill GS, Harris DW, Huff RM, Eaton MJ, Lookingland K et al (1996). Pharmacological characterization of U-101387, a dopamine D4 receptor-selective antagonist. J Pharmacol Exp Ther 279: 1392–1403.
Mohila CA, Onn S-P (2005). Increases in the density of parvalbumin-immunoreactive neurons in the anterior cingulate cortex of amphetamine-withdrawn rats: evidence for corticotropin-releasing factor in sustained elevation. Cerebral Cortex 15: 250–261.
Mrzljak L, Bergson C, Pappy M, Huff R, Leverson R, Goldman-Rakic PS (1996). Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature 381: 245–248.
Onn S-P, Fienberg AA, Grace AA (2003). Dopamine modulation of membrane excitability in striatal spiny neurons is altered in DARPP-32 knockout mice. J Pharm Exp Therapeut 306: 870–879.
Onn S-P, Grace AA (1999). Dopamine modulation of afferent-evoked synaptic responses in identified PFC pyramidal neurons: correspondence with axonal morphology. Soc Neurosci Abstr 664.6; p 1659.
Onn S-P, Grace AA (2000). Amphetamine withdrawal alters bistable states and cellular coupling in rat prefrontal cortex and nucleus accumbens neurons recorded in vivo. J Neurosci 20: 2332–2345.
Onn S-P, Pucak ML, Grace AA (1993). Lucifer yellow dye labeling of living nerve cells and subsequent immunoperoxidase staining with Lucifer yellow antiserum. Neurosci Protocols 90: 153–160.
Patel S, Freedman S, Chapman KL, Emms F, Fletcher AE, Knowles M et al (1997). Biological profile of L-745870, a selective antagonist with high affinity for the dopamine D4 receptor. J Pharmacol Exp Ther 283: 636–647.
Penit-Soria J, Audinat E, Crepel F (1987). Excitation of rat prefrontal cortical neurons by dopamine: an in vitro electrophysiological study. Brain Res 425: 263–274.
Price CJ, Pittman QJ (2001). Dopamine D4 receptor activation inhibits presynaptically glutamatergic neurotransmission in the rat supraoptic nucleus. J Neurophysiol 86: 1149–1155.
Retaux S, Besson MJ, Penit-Soria J (1991). Synergism between D1 and D2 dopamine receptors in the inhibition of the evoked release of [3H]GABA in the rat prefrontal cortex. Neuroscience 43: 323–329.
Reynolds GP, Mason SL (1995). Absence of detectable striatal dopamine D4 receptors in drug-treated schizophrenia. Eur J Pharmacol 281: R5–R6.
Rose HJ, Metherate R (2001). Thalamic stimulation largely elicits orthodromic, rather than antidromic, cortical activation in an auditory thalamiocoritcal slice. Neuroscience 106: 331–340.
Rubinstein M, Cepeda C, Hurst RS, Flores-Hernandez J, Ariano MA, Falzone TL et al (2001). Dopamine D4 receptor-deficient mice display cortical hyperexcitability. J Neurosci 21: 3756–3763.
Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ (2001a). Dopamine D1/D5 receptormodulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA 98: 301–306.
Seamans JK, Gorelova N, Durstewitz D, Yang CR (2001b). Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci 21: 3628–3638.
Seamans JK, Yang CR (2004). The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74: 1–58.
Seeman P, Guan HC, Van Tol HH (1993). Dopamine D4 receptors elevated in schizophrenia. Nature 365: 441–445.
Sesack SR, Carr DB, Omelchenko N, Pinto A (2003). Anatomical substrates for glutamate–dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci 1003: 36–52.
Shi WX, Zheng P, Liang XF, Bunney BS (1997). Characterization of dopamine-induced depolarization of prefrontal cortical neurons. Synapse 26: 415–422.
Silva LR, Amitai Y, Connors BW (1991). Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science 251: 432–435.
Simley JF, Levy AI, Ciliax BJ, Goldman-Rakic PS (1994). D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA 91: 5720–5724.
Sumiyoshi T, Stockmeier CA, Overholser JC, Thompson PA, Meltzer HY (1995). Dopamine D4 receptors and effects of guanine nucleotides on [3H]raclopride binding in postmortem caudate nucleus of subjects with schizophrenia or major depression. Brain Res 681: 109–116.
Sutor B, Hablitz JJ (1989). EPSPs in rat neocortical neurons in vitro 1. Electrophysiological evidence for two distinct EPSPs. J Neurophysiol 61: 607–620.
Tallerico T, Novak G, Liu IS, Ulpian C, Seeman P (2001). Schizophrenia: elevated mRNA for dopamine D2 (Longer) receptors in frontal cortex. Brain Res Mol Brain Res 87: 160–165.
Tseng KY, O'Donnell P (2004). Dopamine–glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci 24: 5131–5139.
Urban NN, Gonzalez-Burgos G, Henze DA, Lewis DA, Barrionuevo G (2002). Selective reduction by dopamine of excitatory synaptic inputs to pyramidal neurons in primate prefrontal cortex. J Physiol 539: 707–712.
Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB et al (1991). Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350: 610–614.
Wang J, O'Donnell P (2001). D1 dopamine receptors potentiate NMDA-mediated excitability increase in layer V prefrontal pyramidal neurons. Cerebral Cortex 11: 452–462.
Wang X, Zhong P, Yan Z (2002). Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci 22: 9185–9193.
Wedzony K, Chocyk A, Mackowiak M, Fijal K, Czyrak A (2000). Cortical localization of dopamine D4 receptors in the rat brain-immunocytochemical study. J Physiol Parmacol 51: 205–221.
Williams GV, Goldman-Rakic PS (1995). Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376: 572–575.
Yang CR, Seamans JK (1996). Dopamine D1 receptor actions in layers V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci 16: 1922–1935.
Zhang K, Grady CJ, Tsapakis EM, Andersen SL, Tarazi FI, Baldessarini RJ (2004). Regulation of working memory by dopamine D4 receptor in rats. Neuropsychopharmacology 29: 1648–1655.
Zhang Z-W, Deschenes M (1997). Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: a single-cell labeling study. J Neurosci 17: 6365–6379.
Zheng P, Zhang XX, Bunney BS, Shi WX (1999). Opposite modulation of cortical N-methyl-D-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience 91: 527–535.
Zhou FM, Hablitz JJ (1999). Dopamine modulation of membrane and synaptic properties of interneurons in rat cerebral cortex. J Neurophysiol 81: 967–976.
Zhu JJ (2000). Maturation of layer 5 neocortical pyramidal neurons: amplifying salient layer 4 and layer 4 inputs by Ca+2 action potentials in adult rat tuft dendrites. J Physiol (London) 526: 571–587.
Acknowledgements
This work was supported by USPHS MH 63498 (S-P Onn) and MH45156 & MH57440 (AAG). We thank Mr Brian Lowry for providing excellent data acquisition system, the Neuroscope Program. We also acknowledge Dr Kalpana Merchant of Pharmacia-Upjohn Pharmaceuticals for the supply of PNU-101387.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onn, SP., Wang, XB., Lin, M. et al. Dopamine D1 and D4 Receptor Subtypes Differentially Modulate Recurrent Excitatory Synapses in Prefrontal Cortical Pyramidal Neurons. Neuropsychopharmacol 31, 318–338 (2006). https://doi.org/10.1038/sj.npp.1300829
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300829
Keywords
This article is cited by
-
Prefrontal Cortical GABA Transmission Modulates Discrimination and Latent Inhibition of Conditioned Fear: Relevance for Schizophrenia
Neuropsychopharmacology (2014)
-
Moderate exercise and chronic stress produce counteractive effects on different areas of the brain by acting through various neurotransmitter receptor subtypes: A hypothesis
Theoretical Biology and Medical Modelling (2006)