Abstract
Drug cues have been shown to activate brain regions involved in attention, motivation, and reward in addicted users. However, as studies have typically measured responses in only one state (ie drug abstinence), it is unclear whether observed activations represent amplification by abstinence or stable responses. Thus, the present study was designed to evaluate the stability of event-related responses to visual drug cues in dependent smokers (n=13) using event-related functional magnetic resonance imaging measures. Imaging was conducted following smoking as usual and following overnight abstinence, and self-reported craving measures were obtained before, during, and after scanning. Analysis of hemodynamic response (HDR) amplitudes in each of 13 regions of interest revealed larger responses to smoking compared to control cues in ventral anterior cingulate gyrus (vACG) and superior frontal gyrus. Responses to smoking cues in these and all other regions revealed no effects of abstinence/satiety, thus supporting the notion that cue-elicited brain responses are relatively stable. However, while the abstinence manipulation did not alter group-level responses to smoking cues, at the individual level, abstinence-induced changes in craving (abstinence minus satiety) were positively correlated with changes in HDR amplitude to smoking cues in frontal regions including left inferior frontal gyrus, left vACG, and bilateral middle frontal gyrus. These results suggest that brain responses to smoking cues, while relatively stable at the group level following short-term abstinence, may be modulated by individual differences in craving in response to abstinence—particularly in regions subserving attention and motivation.
Similar content being viewed by others
INTRODUCTION
While nicotine is thought to be the primary psychoactive agent responsible for cigarette addiction, recent evidence suggests that conditioned sensory cues also play an important role in the maintenance of smoking behavior (Caggiula et al, 2001, 2002; Rose et al, 2000, 2003; Rose and Levin, 1991). Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies of smokers have found cue-induced increases in brain activity in regions associated with attention, motivation, and reward. A PET study comparing responses to smoking vs nature and neutral stimuli found cue-induced increases in glucose metabolism in perigenual anterior cingulate gyrus (ACG), orbitofrontal cortex (OFC), and temporal lobe in smokers (Brody et al, 2002). In an event-related fMRI study (Due et al, 2002), cue-induced responses to smoking-related pictures were observed in regions including medial thalamus, right posterior amygdala, bilateral middle frontal gyrus (MFG), and right inferior frontal gyrus (IFG). These findings in smokers are largely consistent with cue–reactivity neuroimaging studies of other drugs of abuse (Childress et al, 1999; Garavan et al, 2000; George et al, 2001; Grant et al, 1996; Maas et al, 1998; Wang et al, 1999; Wexler et al, 2001; Wrase et al, 2002).
While these previous imaging studies demonstrate increased brain activation in response to drug cues in addicted users vs control subjects, it is unclear whether cue-elicited activations in addicts are stable responses since these studies have examined responses under only one drug condition: abstinence (typically overnight). Thus, the present study sought to assess event-related brain responses to smoking cues in dependent smokers following overnight abstinence and following smoking as usual (satiety). We hypothesized that consistent with positive-incentive models of craving (Robinson and Berridge, 1993), compared to satiety, overnight abstinence would increase craving and result in increased brain activity in response to smoking cues, particularly in regions associated in previous studies with attention (eg ACG), reward (eg nucleus accumbens), and craving (eg insula). We tested this hypothesis by the novel examination of relations between self-reported craving and event-related brain activation rather than brain responses summated over the course of minutes as has been the case in PET studies (Brody et al, 2002). We predicted that abstinence-induced increases in self-reported craving would be correlated with abstinence-induced increases in brain activation in response to smoking cues.
SUBJECTS AND METHODS
Subjects
A total of 16 (n=16) adult dependent smokers were recruited from the community using fliers and newspaper advertisements. In order to participate, subjects had to smoke at least 15 cigarettes per day for at least 2 years, have an afternoon breath carbon monoxide (CO) level greater than 15 ppm, be right-handed, and be free of serious health problems and medications altering CNS functioning. Three subjects were excluded due to excessive head motion (see the Imaging data analysis section) and thus the final sample consisted of 13 smokers (eight females; six minority). Mean subject age was 29.9 years (SD=8.4). They smoked an average of 25.4 cigarettes per day (SD=6.6) with Federal Trade Commission nicotine deliveries of 1.1 mg (SD=0.19) for 13.4 years (SD=6.8). Their breath CO level at screening was 27.8 ppm (SD=0.78). Subjects were compensated with $200 for completing the study.
Design
All subjects completed three sessions: a 1 h screening/practice session and two 2 h fMRI sessions. At the beginning of the screening/practice session, subjects heard a complete description of the study and read and signed an Institutional Review Board-approved informed consent form. They then completed questionnaires regarding health, mood, smoking history, and suitability for fMRI research, and provided a breath sample. They also completed a practice version of the experimental task in a mock fMRI scanner.
Subjects completed two fMRI sessions that were identical except that prior to one session they were allowed to smoke their usual number of cigarettes up until entering the scanning facility (satiated condition) while in the other session they were required to be overnight abstinent from smoking (abstinent condition). Order of condition was randomly assigned and counterbalanced. Compliance with instructions to smoke or not was verified with breath CO levels.
All scanning was conducted between 0800 and 1300. At the beginning of each fMRI session, subjects completed psychometric measures of self-reported craving, provided breath samples, and were escorted to the scanning facility. After subjects were positioned in the fMRI scanner, sets of MRI images were acquired that provided information about brain anatomy and function (described below). At the midpoint of the functional series, an orally presented version of a mood and craving questionnaire was administered. Following all scanning, mood and withdrawal questionnaires were again completed.
Cue Exposure Task
Photographic smoking, control, and target cues were presented during functional imaging. Smoking cues (n=60) consisted of pictures of smoking-related objects and people smoking cigarettes. Control cues (n=60) consisted of pictures of everyday objects (eg stapler, keys) and people engaged in everyday activities (eg using the phone). Target cues (n=15) were pictures of animals. Subjects were asked to press a button whenever they saw a target. Stimuli included pictures from the International Smoking Image Series (Gilbert and Rabinovich, 2003), the International Affective Picture System (Lang et al, 1995), and from a proprietary set. Judges familiar with the aims of the study verified that the smoking and control images were neutral in affect and were balanced in terms of composition, gender, race depicted, and complexity.
All visual stimuli were projected onto a screen behind the subject's head, which the subject viewed using mirrored goggles. During each of the nine experimental runs, 15 images were presented with approximately half being smoking cues, half control cues, and 1–2 targets. Thus, a total of 135 cues were presented during each session, although the order of presentation was varied between days. Picture cues were presented for 4 s with a variable 18–22 s stimulus onset asynchrony.
Imaging Parameters
A 1.5 T GE NVi SIGNA scanner with 41 mT/m gradients was used for image acquisition. Each subject's head was held in place using a vacuum-pack system to minimize head motion. Following a localizer series, BOLD functional images were collected for 28 contiguous slices (4 mm thick) parallel to the horizontal plane connecting the anterior and posterior commissures. A spiral-out gradient-echo pulse sequence sensitive to blood oxygen level-dependent (BOLD) contrast was used, with TR=2000 ms, TE=40 ms, FOV=25.6 cm, matrix=64 × 64, flip angle=90°, and in-plane resolution=4 mm2. After completion of nine runs of the functional data collection, T1-weighted structural imaging was conducted on the same slices as the functional images (ie 28 slices, 4 mm thick) with TR=450 ms, TE=20 ms, FOV=25.6 cm, matrix=256 × 256, and in-plane resolution=1 mm2. Structural images were acquired following functional images in order to minimize the time between the last cigarette and viewing of smoking cues in the satiated condition.
Imaging Data Processing
Prior to data analysis, functional data underwent preprocessing using SPM 99 and data analysis using custom MATLAB (Mathworks Inc.) scripts. Data were corrected via sinc interpolation for time of acquisition of each slice within the imaging volume and were realigned to correct for head motion over the course of the session using SPM 99. Subjects exhibiting head motion in excess of 8 mm in any plane, in either session (n=3), were excluded from further analysis. We then used custom MATLAB scripts to extract epochs surrounding individual events from the overall time series. For each event, we calculated the change in BOLD signal over an 18 s peristimulus epoch (10 TRs: −4 s to +14 s), baseline corrected to the mean value of the first three time points in the epoch. This provided the mean BOLD response to each trial type for each voxel in each subject.
Anatomical regions of interest (ROIs) were drawn bilaterally using two atlases as guides (Duvernoy, 1999; Mai et al, 2003) for the following brain structures: amygdala, hippocampus, ventral and dorsal anterior cingulate, thalamus, caudate, putamen, insula, OFC, and IFG, MFG and superior frontal gyrus (SFG). Given the relatively low resolution of the anatomical images along the Z dimension, the boundaries of the nucleus accumbens were difficult to identify in all subjects. Thus, a ‘ventral striatum’ ROI was drawn, which included the most ventral aspects of the caudate and putamen. Within each of these ROIs, we calculated the mean BOLD response across all voxels within the epochs described in the previous paragraph. These mean ROI responses (for each subject) formed the basis for our data analysis.
Imaging Data Analysis
All analyses were conducted using SPSS for Windows (version 11.5). In order to assess the effects of abstinence/satiety on responses to smoking and control cues, percent signal values from the 4, 6, 8, and 10 s time points (ie those around the predicted maximum of the BOLD response) were entered into separate 2 (Session: abstinent, satiated) × 2 (Stimulus: smoking, control) × 2 (Hemisphere: left, right) × 4 (Time: 4, 6, 8, 10 s) repeated-measures ANOVAs for each ROI. Greenhouse–Giesser correction was applied to effects involving Time, although uncorrected degrees of freedom are reported for clarity.
Because targets were included to control for subjects' attention to the task, and thus have different cognitive and response requirements than the smoking/control cues, we do not show the mean target activation in figures and excluded targets from the ANOVA analyses. However, hemodynamic responses (HDRs) to target cues were robust. For instance, on the satiated day, mean percent signal increases between 4 and 10 s following stimulus onset in the dorsal ACG were 0.19, 0.06, and 0.04% for target, smoking, and control cues, respectively.
To evaluate relations between self-reported craving and responses to cues, difference scores were calculated (abstinence−satiated) for the behavioral measure of self-reported craving and for the brain measures of BOLD activation to smoking and control cues.
Questionnaires
Subjects completed the Shiffman/Jarvik Withdrawal Questionnaire (SWQ) (Shiffman and Jarvik, 1976) at the beginning and end of each scanning session. The SWQ is a 32-item scale measuring nicotine withdrawal symptoms in the following dimensions: Craving (eg ‘Do you have an urge to smoke a cigarette?’), Negative Affect (eg ‘Do you feel tense?’), Arousal (eg ‘Do you feel wide awake?’), Appetite (eg ‘Do you feel hungrier than usual?’), Habit Withdrawal (eg ‘Do you miss having something to do with your hands?’), and Somatic Symptoms (eg ‘Is your heart being faster than usual?’). Items were rated on a seven-point scale ranging from 1 (‘not at all’) to 7 (‘extremely’). Mean item responses for each scale were entered into 2 (Session: abstinent, satiated) × 2 (Time: prescan, postscan) ANOVA conducted for each scale.
A short, nine-item form of the SWQ questionnaire using the same scale as the longer form was administered orally over the intercom midway through scanning sessions in order to assess Craving (three items), Negative Affect (three items), and Arousal (three items). Mean item responses for each scale were entered into separate one-way ANOVAs (Session: abstinent, satiated).
RESULTS
Abstinence Verification
All participants' breath CO levels were less than 15 ppm on the abstinent session day (M=9.46, SD=2.85) and were indicative of overnight abstinence. Breath CO levels on the satiated day (M=26.46 ppm, SD=5.49) were significantly higher (F=135.25, df=1, 12, p<0.0001).
Self-Report Data
Overnight abstinence from smoking (Figure 1) resulted in significantly greater cigarette craving (F=40.15, df=1, 12, p<0.0001), negative affect (F=27.37, df=1, 12, p<0.0001), somatic symptoms (F=9.90, df=1, 12, p=0.008), and habit withdrawal (F=6.62, df=1, 12, p=0.024), as well as significantly lower arousal (F=15.63, df=1, 12, p=0.002). Session × Time interactions were observed for cigarette craving (F=17.00, df=1, 12, p=0.001), negative affect (F=7.97, df=1, 12, p=0.015), and habit withdrawal (F=8.22, df=1, 12, p=0.014), which indicated that symptom levels increased over the course of the satiated session. Subjects reported greater craving during scans (F=21.60, df=1, 12, p=0.001) within the abstinent session compared to the satiated session.
Effects of overnight abstinence and time (prescanning vs postscanning) on mean withdrawal symptoms as measured by SWQ (Shiffman and Jarvik, 1976).
fMRI Results
Shown in Table 1 and Figure 2 are the results from statistical analysis of activation to smoking and control cues. Across sessions, activation was significantly greater over the peak 4–10 s interval to smoking cues than control cues in both SFG (0.063 vs 0.007%) and ventral ACG (0.013 vs −0.037%). A similar and nearly significant effect was observed in ventral striatum (0.036 vs −0.016%). Across cue types, abstinent session responses were significantly greater than satiated session responses in SFG (0.065 vs 0.006%) and caudate (0.014 vs −0.021%) and marginally greater in MFG (0.093 vs 0.023%).
Group-averaged event-related HDRs to smoking and control cues following overnight abstinence (Abstinent) and smoking as usual (Satiated) in dependent smokers. Values are expressed as percent signal change from baseline for the following regions: IFG, MFG, SFG, ventral ACG (V-ACG), dorsal ACG (D-ACG), ventral striatum (V-STR), thalamus (THL), and insula (INS). aObserved effects of Session in MFG and Stimulus in V-STR were only marginally significant (see Table 1 for statistical information).
Session × Stimulus interactions were observed in thalamus, dorsal ACG, and insula. Post hoc analyses of these interactions indicated that, in all three ROIs, responses to control cues during the satiated session were of significantly lower amplitude (all p's<0.05) than (1) responses to smoking cues during the satiated session and (2) responses to control cues during the abstinent session. There were no differences in responses to smoking and control cues during the abstinent session in these regions.
In order to test the hypothesis that smoking abstinence would be associated with larger responses to smoking cues, planned comparisons of responses to smoking cues during the two sessions were conducted upon all ROIs. However, at no ROI were these differences significant.
Laterality effects were observed in several regions. HDRs were higher in amplitude in the left than right hemisphere in SFG (F=9.44, df=1, 12, p=0.01; 0.058 vs 0.012%) and to a lesser degree in thalamus (F=6.79, df=1, 12, p=0.023; 0.042 vs 0.037%). A Side × Session interaction (F=5.09, df=1, 12, p=0.043) in the ventral striatum was indicative of significantly greater (p=0.03) HDR in the right hemisphere during the abstinent session. No Hemisphere × Stimulus interactions were observed.
Craving Correlates
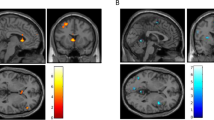
Correlations were calculated between abstinence-induced changes in self-report craving during scanning and brain responses to smoking and control cues (Table 2). Significant positive correlations between changes in craving and responses to smoking cues were found for left IFG, MFG bilaterally (Figure 3), left SFG, left ventral ACG, right dorsal ACG, and left thalamus. For control cues, a negative correlation was observed only for right caudate.
Relations between the effect of overnight abstinence on Shiffman–Jarvik short form Craving and HDRs to smoking cues in the left and right MFG. The effects of overnight abstinence were calculated by subtracting values obtained following smoking as usual from those obtained following overnight abstinence. Positive numbers reflect abstinence-induced increases in craving and larger response to smoking cues.
DISCUSSION
Regardless of smoking abstinence or satiety, event-related HDRs to smoking cues were larger than responses to control cues in regions subserving emotion (ventral ACG), attention (SFG), and marginally, reward (ventral striatum). In addition, overnight abstinence from smoking resulted in both significant withdrawal symptoms and systematic changes in event-related brain responses to smoking and control cues. Responses to control cues were larger following abstinence in regions associated with attention and sensory integration (eg dorsal ACG, thalamus, and insula); however, contrary to our hypotheses, responses to smoking cues were largely unaltered by overnight abstinence. Nonetheless, abstinence-induced changes in self-reported craving were positively correlated with changes in responses to smoking cues but not control cues in frontal cortical regions including frontal and anterior cingulate gyri and in thalamus.
The observation in the present study of greater responses to smoking relative to control cues in ventral ACG across abstinence/satiety is consistent with other recent findings showing involvement of the ACG, and the ventral ACG in particular, in conditioned drug cue processing. In a PET study, Brody et al (2002) observed greater cue-induced increases in glucose metabolism in ventral but not dorsal regions of the ACG in satiated smokers compared to a group of nonsmoker controls. Further, a number of studies, which have not separately examined ventral and dorsal ACG, have observed cue-induced increases in ACG activation (Childress et al, 1999; Garavan et al, 2000; Kilts et al, 2001; Maas et al, 1998; Wexler et al, 2001). These findings are consistent with views that the ACG supports attention and stimulus evaluation—processes likely active during exposure to drug cues. Greater cue-induced activation in ventral as compared to dorsal ACG is also consistent with a meta-analysis of ACG imaging findings (Bush et al, 2000) demonstrating greater involvement of anterior/ventral regions of the ACG in emotional as compared to cognitive processes.
The observation of greater frontal activation to smoking cues is also consistent with prior results. In another event-related fMRI study using a similar task (Due et al, 2002), greater activation was observed in response to smoking cues in IFG and MFG in a group of overnight abstinent smokers but not a nonsmoker control group. While activation in ROIs within SFG was not examined by Due et al, the combined findings of this and the present study suggest that frontal brain regions likely play an important role in the processing of visual drug cues.
Compared to the satiated condition, abstinence did not result in larger responses to smoking cues in any of the ROIs examined. This finding runs counter to positive-incentive models of drug craving (Robinson and Berridge, 1993), which predict that drug abstinence should enhance the perceived incentive quality of cues previously associated with a drug. However, these findings are consistent with a number of studies showing abstinence-induced increases in drug craving but failing to find evidence that short-term smoking abstinence selectively amplifies self-report and physiological responses to conditioned drug cues (Drobes and Tiffany, 1997; Tiffany et al, 2000).
One possible explanation for our not finding changes in brain responses to smoking cues is that responses to conditioned drug cues may, within certain parameters, be fairly stable given the large number of pairings of the cues (eg seeing a lit cigarette) with drug administration (eg nicotine). Thus, while we might hypothesize that longer term abstinence would attenuate brain responses to drug cues, the relatively short-term abstinence manipulation in the present study was likely not long enough to result in significant extinction of conditioned responses and thus attenuate brain activation evoked by these cues.
The present results do not, however, provide definitive evidence that craving does not result in increased drug cue salience. While subjects in the study reported significantly greater craving following abstinence, the difference in craving between the two sessions was minimized by the end of scanning due to an increase in craving over the course of the satiated session. While attempts were made to minimize craving on the satiated day by allowing subjects to smoke 10–20 min before scanning and by delaying anatomical imaging until the end of the session, subjects nevertheless reported significant craving during both sessions—which may account for similar responses to smoking cues across sessions. Future studies in which craving is attenuated to a greater degree by either nicotine replacement or smoking while in the scanner will provide a better assessment of the effects of abstinence.
While the hypothesis that abstinence would increase the amplitude of cue-induced responses was not supported, hypothesized positive correlations between abstinence-induced changes in self-reported craving and responses to smoking cues were observed. Correlations were highest in frontal regions, and were more frequently observed in left (IFG, MFG, SFG, and ventral ACG) than right (MFG and dorsal ACG) regions. Relations between drug cue-induced brain responses and craving self-reports have been reported previously in both smoking and cocaine abusing populations (Bonson et al, 2002; Brody et al, 2002; Kilts et al, 2001; Maas et al, 1998; Wang et al, 1999) with a large degree of agreement across studies. Implicated regions have included orbitofrontal, prefrontal, and insular cortex, and amygdala. For instance, in a PET study of smokers, Brody et al (2002) reported correlations between craving and cue-induced changes in brain metabolism in parts of dorsolateral prefrontal cortex roughly corresponding to the MFG ROI as defined in the present study. In a PET study of cocaine addicts, Bonson et al (2002) observed positive correlations between craving and glucose metabolism in right SFG, left OFC, left amygdala, left posterior insula, and left superior temporal gyrus. Finally, left hemisphere activation has been associated with appetitive motivation broadly and with approach toward drug cues more specifically (Zinser et al, 1999). Thus the present findings are largely consistent with other studies that have observed correlations between responses to cues and activity in frontal and predominantly left hemisphere regions. It should be noted, however, that while correlations between craving and brain responses to smoking cues were lateralized in the present study, we did not find evidence of hemispheric differences in brain activation in response to smoking cues themselves.
The positive correlations observed between self-reported craving and responses to smoking but not control cues can be interpreted in several ways. Greater craving on the abstinent day may have resulted in an increase in the salience or motivational significance of smoking cues, resulting in larger responses to these cues in regions subserving motivation, planning, and attention. Another possibility is that exposure to smoking cues following overnight abstinence resulted in cue-induced craving, which in turn led to larger responses to smoking-relevant stimuli. Future studies assessing a broader range of craving manipulations may help pinpoint the causal links between subjective craving and responses to drug cues.
At first glance, the finding of significant correlations between cue-induced craving and responses to smoking cues appears to be at odds with finding no effect of abstinence/satiety on brain responses to smoking cues. Subjects in the present study showed a distribution of brain responses to smoking cues—some subjects exhibited abstinence-induced increases in event-related brain response amplitudes, while others exhibited decreases. When summed together, these diverse responses resulted in no change from the satiated condition at the group level. As with brain responses, subjects exhibited diverse craving responses to overnight abstinence—with a few subjects actually showing decreased craving. The degree to which overnight abstinence resulted in increased or decreased craving was correlated with the degree to which overnight abstinence resulted in increased or decreased activation in response to smoking cues. Thus, while at the group mean level the results do not support positive-incentive models of drug craving, correlational data suggest that craving and cue-elicited responses are related.
Finally, responses to control cues, which we hypothesized would be unaltered by smoking abstinence, were significantly larger during the deprived session in regions including thalamus, dorsal ACG, and insula. As these regions generally subserve sensory integration and cognitive processing, the change in their activity in response to control cues may reflect several factors. Content and emotional valence were carefully balanced, so that the only difference between smoking and control cues was whether smoking-related or everyday objects were present. While this approach had the advantage of offering tight control over stimulus effects, it potentially might have made discrimination of smoking vs control cues difficult, thus requiring significant information processing resources to make these discriminations. This explanation is consistent with the literature showing that smokers experience decrements in cognitive performance during withdrawal (Bell et al, 1999; Heishman et al, 1994). However, were nicotine withdrawal solely responsible for the observed greater responses to control cues during the abstinent day, one might expect positive correlations between withdrawal symptoms and responses to control cues. As such, no correlations were observed.
The current study has several potential limitations. First, since subjects were unable to smoke in the scanner, self-report craving increased over the course of the satiated session, which, as noted above, may have limited the effects of the abstinence/satiety manipulation on event-related brain responses. Future studies might have subjects smoke or receive nicotine so as to minimize increases in withdrawal. Additionally, the current study is limited in some of the same ways as other fMRI studies. Artifacts associated with magnetic susceptibility differences at air/tissue interfaces may account for a lack of findings in the OFC—a region associated with reward and motivation (London et al, 2000), which has been shown to be active in PET cue–reactivity studies (Bonson et al, 2002; Grant et al, 1996). Also like many fMRI studies, ours was limited by a small sample size and relatively large number of statistical tests. However, the use of an ROI-based approach, rather than a voxel-wise approach, to data analysis should have minimized the probability of Type I error by reducing the overall number of statistical tests (Huettel et al, 2004). Finally, the resolution (voxels=4 mm3) of functional images in the present study made analysis of small yet relevant regions (eg ventral tegmental area) impossible and may have reduced the reliability of results of other regions (eg ventral striatum).
Nevertheless, the study is also characterized by several strengths. Training subjects using a simulated scanner had two salutary effects: it minimized loss of data to head motion artifact and ameliorated learning effects typical to the first fMRI session in a multiple-session study. Utilization of a within-subjects design not only increased statistical power, but also allowed for the examination of correlations between changes in craving and responses to smoking cues across sessions. And, it is the first study to evaluate the effects of a craving manipulation on event-related fMRI responses to drug cues in smokers. Future studies will evaluate the effects of other craving manipulations and treatments designed to reduce craving on cue-induced brain responses.
References
Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ (1999). Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine Tob Res 1: 45–52.
Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL et al (2002). Neural systems and cue-induced cocaine craving. Neuropsychopharmacology 26: 376–386.
Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG et al (2002). Brain metabolic changes during cigarette craving. Arch Gen Psychiatry 59: 1162–1172.
Bush G, Luu P, Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222.
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA et al (2001). Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70: 515–530.
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA et al (2002). Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 163: 230–237.
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18.
Drobes DJ, Tiffany ST (1997). Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol 106: 15–25.
Due DL, Huettel SA, Hall WG, Rubin DC (2002). Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry 159: 954–960.
Duvernoy HM (1999). The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, and Blood Supply, 2nd edn. Springer-Wien: New York, NY.
Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ et al (2000). Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157: 1789–1798.
George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP et al (2001). Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry 58: 345–352.
Gilbert DG, Rabinovich NE (2003). The Emotional Image Series, Version 1.1 Manual. Unpublished manual. Department of Psychology, Southern Illinois University: Carbondale, IL.
Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C et al (1996). Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93: 12040–12045.
Heishman SJ, Taylor RC, Henningfield JE (1994). Nicotine and smoking: a review of effects on human performance. Exp Clin Psychopharmacol 2: 345.
Huettel SA, Song AW, McCarthy G (2004). Functional Magnetic Resonance Imaging. Sinauer Associates: Sunderland, MA.
Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F et al (2001). Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58: 334–341.
Lang PJ, Bradley MM, Cuthbert BN (1995). International Affective Picture System (IAPS): Technical Manual and Affective Ratings. NIMH Center for the Study of Emotion and Attention, University of FL: Gainesville, FL.
London ED, Ernst M, Grant S, Bonson K, Weinstein A (2000). Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex 10: 334–342.
Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW et al (1998). Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 155: 124–126.
Mai JK, Assheuer JK, Paxinos G (2003). Atlas of the Human Brain, 2nd edn. Academic Press: San Diego, CA.
Robinson TE, Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291.
Rose JE, Behm FM, Westman EC, Bates JE, Salley A (2003). Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol Biochem Behav 76: 243–250.
Rose JE, Behm FM, Westman EC, Johnson M (2000). Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav 67: 71–81.
Rose JE, Levin ED (1991). Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. Br J Addict 86: 605–609.
Shiffman SM, Jarvik ME (1976). Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 50: 35–39.
Tiffany ST, Cox LS, Elash CA (2000). Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol 68: 233–240.
Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR et al (1999). Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 64: 775–784.
Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ et al (2001). Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 158: 86–95.
Wrase J, Grusser SM, Klein S, Diener C, Hermann D, Flor H et al (2002). Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry 17: 287–291.
Zinser MC, Fiore MC, Davidson RJ, Baker TB (1999). Manipulating smoking motivation: impact on an electrophysiological index of approach motivation. J Abnorm Psychol 108: 240–254.
Acknowledgements
We thank Rajendra Morey, MD and Simon Tonev, PhD for technical support, Jonathan Wong and Jim Liu for help with data processing, and David Gilbert, PhD for the use of visual stimuli used in this study. The research was supported by National Institute on Drug Abuse Grant R03DA016212-01 (to Dr McClernon).
Author information
Authors and Affiliations
Corresponding author
Additional information
Parts of this paper were presented in poster format at the meeting of the Society for Research on Nicotine and Tobacco, Scottsdale, AZ in February 2004
Rights and permissions
About this article
Cite this article
McClernon, F., Hiott, F., Huettel, S. et al. Abstinence-Induced Changes in Self-Report Craving Correlate with Event-Related fMRI Responses to Smoking Cues. Neuropsychopharmacol 30, 1940–1947 (2005). https://doi.org/10.1038/sj.npp.1300780
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300780
Keywords
This article is cited by
-
Differences in small-world networks between methamphetamine and heroin use disorder patients and their relationship with psychiatric symptoms
Brain Imaging and Behavior (2022)
-
A methodological checklist for fMRI drug cue reactivity studies: development and expert consensus
Nature Protocols (2022)
-
Short-term nicotine deprivation alters dorsal anterior cingulate glutamate concentration and concomitant cingulate-cortical functional connectivity
Neuropsychopharmacology (2020)
-
Functional brain activation changes associated with practice in delaying smoking among moderate to heavy smokers: study protocol and rationale of a randomized trial (COPE)
Trials (2018)
-
Cortical substrates of cue-reactivity in multiple substance dependent populations: transdiagnostic relevance of the medial prefrontal cortex
Translational Psychiatry (2018)