Abstract
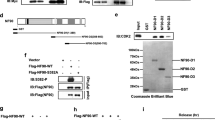
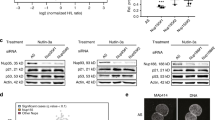
The ribosomal acidic P0 protein, an essential component of the eukaryotic ribosomal stalk, was found to interact with the helix–loop–helix protein human Grap2 and cyclin D interacting protein (GCIP)/D-type cyclin-interacting protein 1/human homolog of MAID protein. Using in vivo and in vitro binding assays, we show that P0 can interact with the N and C termini of GCIP via its N-terminal 39–114 amino-acid residues. Although the P0–GCIP complex was detected mainly in cytoplasmic fraction, polysome profile analysis indicated that the P0–GCIP complex did not coelute with either polysomes or 60S ribosomes, suggesting that GCIP associates with the free form of P0 in the cytoplasm. Transfection of GCIP into MCF-7 cells resulted in decreased levels of pRb phosphorylation. Cotransfection of P0 with GCIP, however, resulted in GCIP-mediated reduction of pRb phosphorylation level which was repressed by P0. Furthermore, overexpression of P0 in breast cancer and hepatocellular cancer cell lines promoted cell growth and colony formation compared to control transfectants. Overexpression of P0 also increased cyclin D1 expression and phosphorylation of pRb at Ser780. Interestingly, P0 mRNA was overexpressed in 12 of 20 pairs of breast cancer/ normal breast specimens (60%). Together, these data indicate that P0 overexpression may cause tumorigenesis in breast and liver tissues at least in part by inhibiting GCIP-mediated tumor suppression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Barnard GF, Staniunas RJ, Bao S, Mafune K, Steele Jr GD, Gollan JL et al. (1992). Increased expression of human ribosomal phosphoprotein P0 messenger RNA in hepatocellular carcinoma and colon carcinoma. Cancer Res 52: 3067–3072.
Chang MS, Chang CL, Huang CJ, Yang YC . (2000). p29, a novel GCIP-interacting protein, localizes in the nucleus. Biochem Biophys Res Commun 279: 732–737.
Diehl JA . (2002). Cycling to cancer with cyclin D1. Cancer Biol Ther 1: 226–231.
Grabowski DT, Pieper RO, Futscher BW, Deutsch WA, Erickson LC, Kelley MR . (1992). Expression of ribosomal phosphoprotein PO is induced by antitumor agents and increased in Mer − human tumor cell lines. Carcinogenesis 13: 259–263.
Hosokawa Y, Arnold A . (1996). Cyclin D1/PRAD1 as a central target in oncogenesis. J Lab Clin Med 127: 246–252.
Kitagawa M, Higashi H, Jung HK, Suzuki-Takahashi I, Ikeda M, Tamai K et al. (1996). The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J 15: 7060–7069.
Kondoh N, Wakatsuki T, Ryo A, Hada A, Aihara T, Horiuchi S et al. (1999). Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res 59: 4990–4996.
Ma W, Stafford LJ, Li D, Luo J, Li X, Ning G et al. (2007). GCIP/CCNDBP1, a helix-loop-helix protein, suppresses tumorigenesis. J Cell Biochem 100: 1376–1386.
Ma W, Xia X, Stafford LJ, Yu C, Wang F, LeSage G et al. (2006). Expression of GCIP in transgenic mice decreases susceptibility to chemical hepatocarcinogenesis. Oncogene 25: 4207–4216.
Remacha M, Jimenez-Diaz A, Santos C, Briones E, Zambrano R, Rodriguez Gabriel MA et al. (1995). Proteins P1, P2, and P0, components of the eukaryotic ribosome stalk. New structural and functional aspects. Biochem Cell Biol 73: 959–968.
Shimizu T, Nakagaki M, Nishi Y, Kobayashi Y, Hachimori A, Uchiumi T . (2002). Interaction among silkworm ribosomal proteins P1, P2 and P0 required for functional protein binding to the GTPase-associated domain of 28S rRNA. Nucleic Acids Res 30: 2620–2627.
Sonnenberg-Riethmacher E, Wustefeld T, Miehe M, Trautwein C, Riethmacher D . (2007). Maid (GCIP) is involved in cell cycle control of hepatocytes. Hepatology 45: 404–411.
Takami T, Terai S, Yokoyama Y, Tanimoto H, Tajima K, Uchida K et al. (2005). Human homologue of maid is a useful marker protein in hepatocarcinogenesis. Gastroenterology 128: 1369–1380.
Tchorzewski M, Krokowski D, Rzeski W, Issinger OG, Grankowski N . (2003). The subcellular distribution of the human ribosomal ‘stalk’ components: P1, P2 and P0 proteins. Int J Biochem Cell Biol 35: 203–211.
Terai S, Aoki H, Ashida K, Thorgeirsson SS . (2000). Human homologue of maid: a dominant inhibitory helix–loop–helix protein associated with liver-specific gene expression. Hepatology 32: 357–366.
Uchiumi T, Kominami R . (1997). Binding of mammalian ribosomal protein complex P0.P1.P2 and protein L12 to the GTPase-associated domain of 28S ribosomal RNA and effect on the accessibility to anti-28S RNA autoantibody. J Biol Chem 272: 3302–3308.
Wool IG, Chan YL, Gluck A, Suzuki K . (1991). The primary structure of rat ribosomal proteins P0, P1, and P2 and a proposal for a uniform nomenclature for mammalian and yeast ribosomal proteins. Biochimie 73: 861–870.
Wu S, Storey KB . (2005). Up-regulation of acidic ribosomal phosphoprotein P0 in response to freezing or anoxia in the freeze tolerant wood frog, Rana sylvatica. Cryobiology 50: 71–82.
Xia C, Bao Z, Tabassam F, Ma W, Qiu M, Hua S et al. (2000). GCIP, a novel human grap2 and cyclin D interacting protein, regulates E2F-mediated transcriptional activity. J Biol Chem 275: 20942–20948.
Yao Y, Doki Y, Jiang W, Imoto M, Venkatraj VS, Warburton D et al. (2000). Cloning and characterization of DIP1, a novel protein that is related to the Id family of proteins. Exp Cell Res 257: 22–32.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Chang, TW., Chen, CC., Chen, KY. et al. Ribosomal phosphoprotein P0 interacts with GCIP and overexpression of P0 is associated with cellular proliferation in breast and liver carcinoma cells. Oncogene 27, 332–338 (2008). https://doi.org/10.1038/sj.onc.1210651
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210651
Keywords
This article is cited by
-
Natural humoral immune response to ribosomal P0 protein in colorectal cancer patients
Journal of Translational Medicine (2015)
-
The OsSec18 complex interacts with P0(P1-P2)2 to regulate vacuolar morphology in rice endosperm cell
BMC Plant Biology (2015)
-
Ribosomal proteins as novel players in tumorigenesis
Cancer and Metastasis Reviews (2013)
-
Proteomic Analysis of Differentially Expressed Proteins in Peripheral Cholangiocarcinoma
Cancer Microenvironment (2011)