Abstract
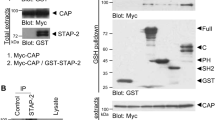
Fms interacting protein (FMIP) is a substrate for Fms tyrosine kinase, and a nuclear/cytoplasm shuttling protein with a leucine zipper. As the phosphorylation of FMIP is observed in insulin-stimulated preadipocytes, we examined the role of FMIP in adipocyte differentiation, using the mesenchymal multipotent stem cells, C2C12 cells, that can differentiate into adipocytes, muscle cells and osteoblasts. Ectopic expression of FMIP in C2C12 impairs the adipocyte differentiation induced by treatment with insulin, dexamethasone and 3-isobutyl-1-methylxanthine. These cells exhibit muscle phenotype with multinuclear morphology. Furthermore, knockdown of endogenous FMIP expression by small interfering RNA improves adipocytic lineage commitment of C2C12 cells, while impairing muscle differentiation. Upon stimulation with insulin, CCAAT/enhancer binding protein (C/EBP)beta, but not C/EBPalpha, is upregulated in cells expressing ectopic FMIP, whereas in FMIP knockdown cells, C/EBPalpha is constitutively expressed. Ectopic expression of C/EBPalpha counteracts the effects of FMIP, whereas C/EBPalpha knockdown partially mimics the effects of FMIP in this system. Northern blot analysis and reverse transcriptase–polymerase chain reaction study reveal that ectopic FMIP-expressing cells do not contain the polyadenylated C/EBPalpha mRNA, but contain the C/EBPalpha pre-mRNA, suggesting that FMIP plays a role in RNA processing and/or export. Indeed, a member of the THO complex that plays a role in mRNA export, THOC1, is co-precipitated with FMIP. The data we have acquired on FMIP suggest that it is a target for tyrosine kinase receptors that potentiate mRNA export.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cao Z, Umek RM, McKnight SL . (1991). Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 5: 1538–1552.
Flodby P, Barlow C, Kylefjord H, Ahrlund-Richter L, Xanthopoulos KG . (1996). Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein alpha. J Biol Chem 271: 24753–24760.
Friedman AD . (2002). Transcriptional regulation of granulocyte and monocyte development. Oncogene 21: 3377–3390.
Freytag SO, Paielli DL, Gilbert JD . (1994). Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev 8: 1654–1663.
Fux C, Mitta B, Kramer BP, Fussenegger M . (2004). Dual-regulated expression of C/EBP-alpha and BMP-2 enables differential differentiation of C2C12 cells into adipocytes and osteoblasts. Nucleic Acids Res 32: e1.
Gridley S, Lane WS, Garner CW, Lienhard GV . (2005). Novel insulin-elicited phosphoproteins in adipocytes. Cell Signaling 17: 59–66.
Helftenbein G, Krusekopf K, Just U, Cross M, Ostertag W, Niemann H et al. (1996). Transcriptional regulation of the c-fms proto-oncogene mediated by granulocyte/macrophage colony-stimulating factor (GM-CSF) in murine cell lines. Oncogene 12: 931–935.
Hu E, Tonzoz P, Spiegelman BM . (1995). Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci USA 92: 9856–9860.
Johnson PF . (2005). Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci 118: 2545–2555.
Jones LC, Lin ML, Chen SS, Klug U, Hofmann WK, Lee S et al. (2002). Expression of C/EBPbeta from the C/ebpalpha gene locus is sufficient for normal hematopoiesis in vivo. Blood 99: 2032–2036.
Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC et al. (2002). A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118.
Koch A, Mancini A, El Bounkari O, Tamura T . (2005). The SH2-domian-containing inositol 5-phosphatase (SHIP)-2 binds to c-Met directly via tyrosine residue 1356 and involves hepatocyte growth factor (HGF)-induced lamellipodium formation, cell scattering and cell spreading. Oncogene 24: 3436–3447.
Lane MD, Tang QQ, Jiang MS . (1999). Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. BBRC 266: 677–683.
Lin FT, Lane MD . (1994). CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci USA 91: 8757–8761.
Mancini A, Koch A, Whetton AD, Tamura T . (2004). The M-CSF receptor substrate and interacting protein FMIP is governed in its subcellular localization by protein kinase C-mediated phosphorylation, and thereby potentiates M-CSF-mediated differentiation. Oncogene 23: 6581–6589.
Mason DY, Cordell JL, Pulford KAF . (1983) In: Bullock GR, Petrusz P (eds). Techniques in Immunocytochemistry. Academic Press: London, pp 175–216.
Masuda S, Das R, Cheng H, Hurt E, Dorman N, Reed R . (2005). Recruitment of the human TREX complex to mRNA during splicing. Gene Dev 19: 512–1517.
Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y et al. (2005). Role of Kruppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J Biol Chem 280: 12867–12875.
Otto TC, Lane MD . (2005). Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol 40: 229–242.
Park BH, Qiang L, Farmer SR . (2004). Phosphorylation of C/EBPbeta at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol Cell Biol 24: 8671–8680.
Popernack PM, Truong LT, Kamphuis M, Henderson AJ . (2001). Ectopic expression of CCAAT/enhancer binding protein beta (C/EBPbeta) in long-term bone marrow cultures induces granulopoiesis and alters stromal cell function. J Hematother Stem Cell Res 10: 631–642.
Rehwinkel J, Herold A, Gari K, Köcher T, Rode M, Ciccarelli FL et al. (2004). Genome-wide analysis of mRNAs regulated by the THO complex in Drosophila melanogaster. Nat Struct Mol Biol 11: 558–566.
Scherr M, Battmer K, Blomer U, Schiedlmeier B, Ganser A, Grez M et al. (2002). Lentiviral gene transfer into peripheral blood-derived CD34+ NOD/SCID-repopulating cells. Blood 99: 709–712.
Scherr M, Battmer K, Ganser A, Eder M . (2003). Modulation of gene expression by lentiviral-mediated delivery of small interfering RNA. Cell Cycle 2: 251–257.
Shi XM, Shi W, Li Q, Song B, Wan M, Bai S et al. (2003). A glucocorticoid-induced leucine-zipper protein. GILZ, inhibits adipogenesis of mesenchymal cells. EMBO Rep 4: 374–380.
Tamura T, Mancini A, Joos H, Koch A, Hakim C, Dumanski J et al. (1999). FMIP, a novel Fms-interacting protein, affects granulocyte/macrophage differentiation. Oncogene 18: 6488–6495.
Tang QQ, Otto TC, Lane MD . (2003). CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci USA 100: 850–855.
Tenen DG, Hromas R, Licht JD, Zhang DE . (1997). Transcription factors, normal myeloid development, and leukemia. Blood 90: 489–519.
Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD et al. (1995). Impaired energy homeostasis in C/EBP alpha knockout mice. Science 269: 1108–1112.
Wang QF, Friedman AD . (2002). CCAAT/enhancer-binding proteins are required for granulopoiesis independent of their induction of the granulocyte colony-stimulating factor receptor. Blood 99: 2776–2785.
Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG . (1997). Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci USA 94: 569–574.
Zhang JW, Tang QQ, Vinson C, Lane MD . (2004). Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci USA 101: 43–47.
Acknowledgements
We thank Regina Wilms, Sabine Klebba-Faerber and Iris Dallmann for technical assistance, Renate Scheibe for providing C2C12 cells and Bruce Boschek for critically reading the manuscript. The research was supported by Sonderforschungsbereich566 (B2) and the Deutsche Forschungsgemeinschaft (Ta-111/8/-4). AHB, KP, ADW and LL are supported by the Leukaemia Research Fund and ADW by BBSRC. This work is a part of PhD thesis of A-FN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mancini, A., El Bounkari, O., Norrenbrock, AF. et al. FMIP controls the adipocyte lineage commitment of C2C12 cells by downmodulation of C/EBPalpha. Oncogene 26, 1020–1027 (2007). https://doi.org/10.1038/sj.onc.1209853
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209853
Keywords
This article is cited by
-
MORC2 regulates C/EBPα-mediated cell differentiation via sumoylation
Cell Death & Differentiation (2019)
-
Prestressed cells are prone to cytoskeleton failures under localized shear strain: an experimental demonstration on muscle precursor cells
Scientific Reports (2018)
-
Oxidative stress-induced S100B accumulation converts myoblasts into brown adipocytes via an NF-κB/YY1/miR-133 axis and NF-κB/YY1/BMP-7 axis
Cell Death & Differentiation (2017)
-
Depletion of three combined THOC5 mRNA export protein target genes synergistically induces human hepatocellular carcinoma cell death
Oncogene (2016)
-
Murine precision-cut liver slices (PCLS): a new tool for studying tumor microenvironments and cell signaling ex vivo
Cell Communication and Signaling (2014)