Abstract
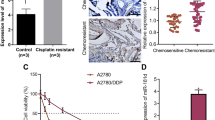
Ovarian cancer is currently the second leading cause of gynecological malignancy and cisplatin or cisplatin-based regimens have been the standard of care for the treatment of advance epithelial ovarian cancers. However, the efficacy of cisplatin treatment is often limited by the development of drug resistance either through the inhibition of apoptotic genes or activation of antiapoptotic genes. We have previously reported the overexpression of human UO-44 (HuUO-44) in ovarian cancers and the HuUO-44 antisera markedly inhibited NIH-OVCAR3 ovarian cancer cell attachment and proliferation (Oncogene 23: 5707–5718, 2004). In the present study, we observed through the cancer cell line profiling array that the expression of HuUO-44 was suppressed in the ovarian cancer cell line (SKOV-3) after treatment with several chemotherapeutic drugs. Similarly, this suppression in HuUO-44 expression was also correlated to the cisplatin sensitivity in two other ovarian cancer cell lines NIH-OVCAR3 and OV-90 in a dose-dependent manner. To elucidate the function of HuUO-44 in cisplatin chemoresistance in ovarian cancer cell, small interfering RNAs (siRNAs) were employed to mediate HuUO-44 silencing in ovarian cancer cell line, NIH-OVCAR3. HuUO-44 RNA interference (RNAi) resulted in the inhibition of cell growth and proliferation. Importantly, HuUO-44 RNAi significantly increased sensitivity of NIH-OVCAR3 to cytotoxic stress induced by cisplatin (P<0.01). Strikingly, we have also demonstrated that overexpression of HuUO-44 significantly conferred cisplatin resistance in NIH-OVCAR3 cells (P<0.05). Taken together, UO-44 is involved in conferring cisplatin resistance; the described HuUO-44-specific siRNA oligonucleotides that can potently silence HuUO-44 gene expression may prove to be valuable pretreatment targets for antitumor therapy or other pathological conditions that involves aberrant HuUO-44 expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chen D, Xu XP, Zhu LJ, Angervo M, Li QX, Bagchi MK et al. (1999). Cloning and uterus/oviduct-specific expression of a novel estrogen-regulated gene (ERG-1). J Biol Chem 274: 32215–32224.
Choudhury A, Charo J, Parapuram SK, Hunt RC, Hunt DM, Seliger B et al. (2004). Small interfering RNA (siRNA) inhibits the expression of the Her2/Neu gene, upregulates HLA Class I and induces apoptosis of Her2/Neu positive tumor cell lines. Int J Cancer 108: 71–77.
Cioca DP, Aoki Y, Kiyosawa K . (2003). RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Ther 10: 125–133.
Devi GR . (2006). SiRNA-based approaches in cancer therapy. Cancer Gene Ther (in press).
Duan Z, Brakora KA, Seiden MV . (2004). Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther 3: 833–838.
Frisch SM, Francis H . (1994). Disruption of epithelial cell–matrix interactions induces apoptosis. J Cell Biol 124: 619–626.
Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K . (2001). Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci 114: 4557–4565.
Huynh H, Ng CY, Lim KB, Ong CK, Ong CS, Tran E et al. (2001). Induction of UO-44 gene expression by tamoxifen in the rat uterus and ovary. Endocrinology 142: 2985–2995.
Imamura T, Asada M, Vogt SK, Rudnick DA, Lowe ME, Muglia LJ . (2002). Protection from pancreatitis by the zymogen granule membrane protein integral membrane-associated protein-1. J Biol Chem 277: 50725–50733.
Judson PL, Watson JM, Gehrig PA, Fowler Jr WC, Haskill JS . (1999). Cisplatin inhibits paclitaxel-induced apoptosis in cisplatin-resistant ovarian cancer cell lines: possible explanation for failure of combination therapy. Cancer Res 59: 2425–2432.
July LV, Beraldi E, So A, Fazli L, Evans K, English JC et al. (2004). Nucleotide-based therapies targeting clusterin chemosensitize human lung adenocarcinoma cells both in vitro and in vivo. Mol Cancer Ther 3: 223–232.
Kasik JW . (1998). A cDNA cloned from pregnant mouse uterus exhibits temporo-spatial expression and predicts a novel protein. Biochem J 330: 947–950.
Leong CT, Ng CY, Ong CK, Ng CP, Ma ZS, Nguyen TH et al. (2004). Molecular cloning, characterization and isolation of novel spliced variants of the human ortholog of a rat estrogen-regulated membrane-associated protein, UO-44. Oncogene 23: 5707–5718.
McGuire III WP, Markman M . (2003). Primary ovarian cancer chemotherapy: current standards of care. Br J Cancer 89 (Suppl 3): S3–S8.
Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R et al. (2004). Inhibition of fatty acid synthase (FAS) suppresses HER2/Neu (ErbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci USA 101: 10715–10720.
Miyamoto S, Hirata M, Yamazaki A, Kageyama T, Hasuwa H, Mizushima H et al. (2004). Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Res 64: 5720–5727.
Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL et al. (2000). Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol 18: 106–115.
Ryther RC, Flynt AS, Phillips III JA, Patton JG . (2005). SiRNA therapeutics: big potential from small RNAs. Gene Ther 12: 5–11.
Siddik ZH . (2003). Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22: 7265–7279.
Tebes SJ, Kruk PA . (2005). The genesis of RNA interference, its potential clinical applications, and implications in gynecologic cancer. Gynecol Oncol 99: 736–741.
Tuschl T, Zamore PD, Lehmann R, Bartel DP, Sharp PA . (1999). Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev 13: 3191–3197.
van Engeland M, Ramaekers FC, Schutte B, Reutelingsperger CP . (1996). A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 24: 131–139.
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C . (1995). A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods 184: 39–51.
Yang G, Cai KQ, Thompson-Lanza JA, Bast Jr RC, Liu J . (2004). Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/Neu gene expression. J Biol Chem 279: 4339–4345.
Yang G, Thompson JA, Fang B, Liu J . (2003). Silencing of H-Ras gene expression by retrovirus-mediated siRNA decreases transformation efficiency and tumor growth in a model of human ovarian cancer. Oncogene 22: 5694–5701.
Acknowledgements
This work was supported by funding from the National Medical Research Council of Singapore under the Grant NMRC/0887/2004 awarded to Huynh Hung.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Leong, C., Ong, C., Tay, S. et al. Silencing expression of UO-44 (CUZD1) using small interfering RNA sensitizes human ovarian cancer cells to cisplatin in vitro. Oncogene 26, 870–880 (2007). https://doi.org/10.1038/sj.onc.1209836
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209836
Keywords
This article is cited by
-
Oncogenes associated with drug resistance in ovarian cancer
Journal of Cancer Research and Clinical Oncology (2015)
-
Androgen Receptor Protein Levels Are Significantly Reduced in Serous Ovarian Carcinomas Compared with Benign or Borderline Disease but Are Not altered by Cancer Stage or Metastatic Progression
Hormones and Cancer (2013)
-
Targeted delivery of microRNA-145 to metastatic breast cancer by peptide conjugated branched PEI gene carrier
Macromolecular Research (2013)