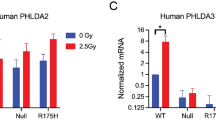
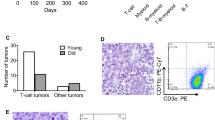
Abstract
The cell cycle inhibitor p21Waf1/Cip1 is among the most important mediators of the tumor suppressor p53. However, there is increasing evidence indicating that p21 could favor tumorigenesis in specific cell types. In particular, the absence of p21 delays the development of thymic lymphomas induced either by ataxia-telangiectasia mutated deficiency or by ionizing irradiation. Here, we extend these observations to the context of p53-deficient mice. The absence of p21 results in a significant extension of the lifespan of p53-null and p53-haploinsufficient mice, and this effect can be attributed exclusively to a decrease in the incidence of spontaneous thymic lymphomas. Specifically, despite the occurrence of a variety of tumor types in the context of p53 deficiency, the only tumors that were significantly impaired by the absence of p21 were thymic lymphomas. Moreover, the absence of p21 also delays the incidence of radiation-induced thymic lymphomas in p53-deficient mice. Interestingly, p21-deficient lymphomas have a higher apoptotic rate than p21-proficient lymphomas, and this could be on the basis of the delayed incidence of thymic lymphomas in the absence of p21. Together, our results indicate that p21 plays an oncogenic role restricted to thymic lymphomas that is mechanistically independent of p53 and associated to a lower tumor apoptotic rate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Adnane J, Jackson RJ, Nicosia SV, Cantor AB, Pledger WJ, Sebti SM . (2000). Oncogene 19: 5338–5347.
Balomenos D, Martin-Caballero J, Garcia MI, Prieto I, Flores JM, Serrano M et al. (2000). Nat Med 6: 171–176.
Bearss DJ, Lee RJ, Troyer DA, Pestell RG, Windle JJ . (2002). Cancer Res 62: 2077–2084.
Brugarolas J, Bronson RT, Jacks T . (1998). J Cell Biol 141: 503–514.
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ . (1995). Nature 377: 552–557.
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P . (1995). Cell 82: 675–684.
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM et al. (1993). Cell 75: 817–825.
Franklin DS, Godfrey VL, O'Brien DA, Deng C, Xiong Y . (2000). Mol Cell Biol 20: 6147–6158.
Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM et al. (2002). EMBO J 21: 6225–6235.
Gartel AL, Tyner AL . (2002). Mol Cancer Ther 1: 639–649.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ . (1993). Cell 75: 805–816.
Jackson RJ, Adnane J, Coppola D, Cantor A, Sebti SM, Pledger WJ . (2002). Oncogene 21: 8486–8497.
Jackson RJ, Engelman RW, Coppola D, Cantor AB, Wharton W, Pledger WJ . (2003). Cancer Res 63: 3021–3025.
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T . (1993). Nature 362: 847–849.
Martin-Caballero J, Flores JM, Garcia-Palencia P, Collado M, Serrano M . (2004). Oncogene 23: 8231–8237.
Martin-Caballero J, Flores JM, Garcia-Palencia P, Serrano M . (2001). Cancer Res 61: 6234–6238.
Martins CP, Berns A . (2002). EMBO J 21: 3739–3748.
Philipp J, Vo K, Gurley KE, Seidel K, Kemp CJ . (1999). Oncogene 18: 4689–4698.
Poole AJ, Heap D, Carroll RE, Tyner AL . (2004). Oncogene 23: 8128–8134.
Salvador JM, Hollander MC, Nguyen AT, Kopp JB, Barisoni L, Moore JK et al. (2002). Immunity 16: 499–508.
Suzuki A, Tsutomi Y, Akahane K, Araki T, Miura M . (1998). Oncogene 17: 931–939.
Suzuki A, Tsutomi Y, Yamamoto N, Shibutani T, Akahane K . (1999). Mol Cell Biol 19: 3842–3847.
Topley GI, Okuyama R, Gonzales JG, Conti C, Dotto GP . (1999). Proc Natl Acad Sci USA 96: 9089–9094.
Wang YA, Elson A, Leder P . (1997). Proc Natl Acad Sci USA 94: 14590–14595.
Weinberg WC, Fernandez-Salas E, Morgan DL, Shalizi A, Mirosh E, Stanulis E et al. (1999). Cancer Res 59: 2050–2054.
Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D . (1993). Nature 366: 701–704.
Xu SQ, El-Deiry WS . (2000). Biochem Biophys Res Commun 269: 179–190.
Yang WC, Mathew J, Velcich A, Edelmann W, Kucherlapati R, Lipkin M et al. (2001). Cancer Res 61: 565–569.
Yang W, Velcich A, Lozonschi I, Liang J, Nicholas C, Zhuang M et al. (2005). Am J Pathol 166: 1239–1246.
Acknowledgements
We thank Teresa de la Cueva for the management of the mouse colonies. This work has been supported in part by grants from the Spanish Ministry of Education and Science (to JF-P and to MS), the Lymphoma Network from the Spanish Ministry of Health (to JF-P), and the European Union (INTACT, to MS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De la Cueva, E., García-Cao, I., Herranz, M. et al. Tumorigenic activity of p21Waf1/Cip1 in thymic lymphoma. Oncogene 25, 4128–4132 (2006). https://doi.org/10.1038/sj.onc.1209432
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209432
Keywords
This article is cited by
-
Suppressor of cytokine signaling 1-dependent regulation of the expression and oncogenic functions of p21CIP1/WAF1 in the liver
Oncogene (2016)
-
EGFR and SYNE2 are associated with p21 expression and SYNE2 variants predict post-operative clinical outcomes in HBV-related hepatocellular carcinoma
Scientific Reports (2016)
-
CDKN1A and FANCD2 are potential oncotargets in Burkitt lymphoma and multiple myeloma
Experimental Hematology & Oncology (2015)
-
The leukemia inhibitory factor (LIF) and p21 mediate the TGFβ tumor suppressive effects in human cutaneous melanoma
BMC Cancer (2015)
-
Transcription factors and target genes of pre-TCR signaling
Cellular and Molecular Life Sciences (2015)