Abstract
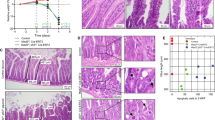
The gene encoding the human BRCA2 tumour suppressor is mutated in a number of different tumour types, most notably inherited breast cancers. The primary role of BRCA2 is thought to lie in the maintenance of genomic stability via its role in the homologous recombination pathway. We generated mice in which Brca2 was deleted from virtually all cells within the adult small intestine, using a CYP1A1-driven Cre-Lox approach. We noted a significant p53-dependent increase in the levels of spontaneous apoptosis which persisted for several months after removal of the gene and ultimately we observed the spontaneous deletion of Brca2-deficient stem cells. Brca2 deficiency did not lead to gross changes in intestinal physiology but did enhance sensitivity to a variety of DNA crosslinking agents. Taken together, our results indicate that Brca2 plays an important role in the response to DNA damage in the small intestine. Furthermore, we show that Brca2 deficiency results in the spontaneous deletion of stem cells, thereby protecting the small intestine against tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Abbott DW, Freeman ML and Holt JT . (1998). J. Natl. Cancer Inst., 90, 978–985.
Chen P-L, Chen C-F, Chen Y, Xiao J, Sharp ZD and Lee W-H . (1998). Proc. Natl. Acad. Sci. USA, 95, 5287–5292.
Cheung AM, Elia A, Tsao MS, Done S, Wagner KU, Hennighausen L, Hakem R and Mak TW . (2004). Cancer Res., 64, 1959–1965.
Cheung AMY, Hande MP, Jalali F, Tsao M-S, Skinnider B, Hirao A, McPherson JP, Karaskova J, Suzuki A, Wakeham A, You-Ten A, Elia A, Squire J, Bristow R, Hakem R and Mak TW . (2002). Cancer Res., 62, 6194–6204.
D'Andrea AD and Grompe M . (2002). Nat. Rev. Cancer, 3, 23–34.
Davies AD, Masson J-Y, McIlwrath MJ, Stasiak AZ, Stasiak A, Venkitaraman AR and West SC . (2001). Mol. Cell, 7, 273–282.
Donoho G, Brenneman MA, Cui TX, Donoviel D, Vogel H, Goodwin EH, Chen DJ and Hasty P . (2003). Genes Chromosomes Cancer, 36, 317–331.
Garcia SB, Park HS, Novelli M and Wright NA . (1999). J. Pathol., 187, 61–81.
Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ and Winton DJ . (2004). Gasteroenterology, 126, 1236–1246.
Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M and Berns A . (2001). Nat. Genet., 29, 418–425.
Kraakman-van der Zwet M, Overkamp WJI, van Lange REE, Essers J, van Duijn-Goedhart A, Wiggers I, Swaminathan S, van Buul PPW, Errami A, Tan RTL, Jaspers NGJ, Sharan SK, Kanaar R and Zdzienicka MZ . (2002). Mol. Cell. Biol., 22, 669–679.
Liu Y and West SC . (2002). Breast Cancer Res., 4, 9–13.
Ludwig T, Chapman D, Papaioannou V and Efstratiadis A . (1997). Genes Dev., 11, 1226–1241.
Ludwig T, Fisher P, Murty V and Efstratiadis A . (2001). Oncogene, 20, 3937–3948.
Marshman E, Booth C and Potten CS . (2002). BioEssays, 24, 91–98.
Marshman E, Ottewell PD, Potten CS and Watson AJ . (2001). J. Pathol., 195, 285–292.
Morimatsu M, Donoho G and Hasty P . (1998). Cancer Res., 58, 3441–3447.
Patel KJ, Yu VPCC, Lee H, Corcoran A, Thistlethwaite FC, Evans MJ, Colledge WH, Friedman LS, Ponder BAJ and Venkitaraman AR . (1998). Mol. Cell, 1, 347–357.
Pellegrini L, Yu DS, Lo T, Anand S, Lee M-Y, Blundell TL and Venkitaraman AR . (2002). Nature, 420, 287–293.
Sansom OJ and Clarke AR . (2002). Oncogene, 21, 5934–5939.
Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR and Winton DJ . (2004). Genes Dev., 18, 1385–1390.
Scully R . (2000). Breast Cancer Res., 2, 324–330.
Sharan SK, Morimatsu M, Albrecht U, Lim D, Regel E, Dinh C, Sands A, Eichele G, Hasty P and Bradley A . (1997). Nature, 386, 804–810.
Soriano P . (1999). Nat. Genet., 21, 70–71.
Suzuki A, de la Pompa JL, Hakem R, Elia A, Yoshida R, Mo R, Nishina H, Chuang T, Wakeham A, Itie A, Koo W, Billia P, Ho A, Fukumoto M, Hui CC and Mak TW . (1997). Genes Dev., 11, 1242–1252.
The Breast Cancer Linkage Consortium (1999). J. Natl. Cancer Inst., 91, 1310–1316.
Acknowledgements
We thank Professor Tak Mak of the University of Toronto, Canada, for the generous donation of mice bearing the floxed Brca2 allele. We also acknowledge Mark Bishop and Derek Scarborough for valuable technical assistance. This work was supported by CR-UK, AICR and Wales Gene Park.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hay, T., Patrick, T., Winton, D. et al. Brca2 deficiency in the murine small intestine sensitizes to p53-dependent apoptosis and leads to the spontaneous deletion of stem cells. Oncogene 24, 3842–3846 (2005). https://doi.org/10.1038/sj.onc.1208533
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208533
Keywords
This article is cited by
-
Brca2 deficiency drives gastrointestinal tumor formation and is selectively inhibited by mitomycin C
Cell Death & Disease (2020)
-
Brf1 loss and not overexpression disrupts tissues homeostasis in the intestine, liver and pancreas
Cell Death & Differentiation (2019)
-
Robust homology-directed repair within mouse mammary tissue is not specifically affected by Brca2 mutation
Nature Communications (2016)
-
Silencing of BRCA2 decreases anoikis and its heterologous expression sensitizes yeast cells to acetic acid-induced programmed cell death
Apoptosis (2014)
-
Myc deletion rescues Apc deficiency in the small intestine
Nature (2007)