Abstract
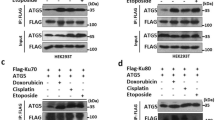
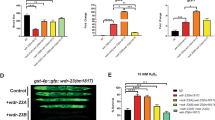
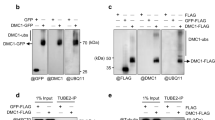
Three of the Rad family proteins, Rad9, Rad1, and Hus1, can interact with each other and form a heterotrimeric complex that is thought to play a role in the sensing step of the DNA integrity checkpoint pathways, but the nature of the Rad9–Rad1–Hus1 complex assembly remains enigmatic. Here, we demonstrate that the human hRad1 protein plays a significant role as molecular chaperone in the process of the hRad9–hRad1–hHus1 heterotrimeric complex formation. In contrast to hRad1, hHus1 is an unstable protein that is actively degraded via the ubiquitin–proteasome pathway. We show that treating cells with proteasome-specific inhibitors stabilizes hHus1 expression. Moreover, hRad1 can associate with hHus1 in the absence of hRad9 and protect hHus1 from ubiquitination and degradation in the cytoplasm. Importantly, genotoxic stress induces hRad1 expression and stabilizes the hHus1 protein. Taken together, these findings suggest a novel role of hRad1 as a potential intrinsic chaperone in the stabilization of hHus1 for the hRad9–hRad1–hHus1 checkpoint complex formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bao S, Tibbetts RS, Brumbaugh KM, Fang Y, Richardson DA, Ali A, Chen SM, Abraham RT and Wang XF . (2001). Nature, 411, 969–974.
Bendjennat M, Boulaire J, Jascur T, Brickner H, Barbier V, Sarasin A, Fotedar A and Fotedar R . (2003). Cell, 114, 599–610.
Bermudez VP, Lindsey-Boltz LA, Cesare AJ, Maniwa Y, Griffith JD, Hurwitz J and Sancar A . (2003). Proc. Natl. Acad. Sci. USA, 100, 1633–1638.
Burtelow MA, Roos-Mattjus PM, Rauen M, Babendure JR and Karnitz LM . (2001). J. Biol. Chem., 276, 25903–25909.
Chen MJ, Lin YT, Lieberman HB, Chen G and Lee EY . (2001). J. Biol. Chem., 276, 16580–16586.
Fink AL . (1999). Physiol. Rev., 79, 425–449.
Foray N, Marot D, Gabriel A, Randrianarison V, Carr AM, Perricaudet M, Ashworth A and Jeggo P . (2003). EMBO J., 22, 2860–2871.
Frydman J . (2001). Annu. Rev. Biochem., 70, 603–647.
Goetz MP, Toft DO, Ames MM and Erlichman C . (2003). Ann. Oncol., 14, 1169–1176.
Griffith JD, Lindsey-Boltz LA and Sancar A . (2002). J. Biol. Chem., 277, 15233–15236.
Griffiths DJ, Barbet NC, McCready S, Lehmann AR and Carr AM . (1995). EMBO J., 14, 5812–5823.
Hirai I and Wang HG . (2002). J. Biol. Chem., 277, 25722–25727.
Hoege C, Pfander B, Moldovan GL, Pyrowolakis G and Jentsch S . (2002). Nature, 419, 135–141.
Kaur R, Kostrub CF and Enoch T . (2001). Mol. Cell. Biol., 12, 3744–3758.
Kondo T, Matsumoto K and Sugimoto K . (1999). Mol. Cell. Biol., 19, 1136–1143.
Lee MW, Hirai I and Wang H-G . (2003). Oncogene, 22, 6340–6346.
Majka J and Burgers PM . (2003). Proc. Natl. Acad. Sci. USA, 100, 2249–2254.
McBride WH, Iwamoto KS, Syljuasen R, Pervan M and Pajonk F . (2003). Oncogene, 22, 5755–5773.
Melo J and Toczyski D . (2002). Curr. Opin. Cell Biol., 14, 237–245.
O'Connell MJ, Walworth NC and Carr AM . (2000). Trends Cell Biol., 10, 296–303.
Post S, Weng YC, Cimprich K, Chen LB, Xu Y and Lee EY . (2001). Proc. Natl. Acad. Sci. USA, 98, 13102–13107.
Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D and Goldberg AL . (1994). Cell, 78, 761–771.
Roos-Mattjus P, Hopkins KM, Oestreich AJ, Vroman BT, Johnson KL, Naylor S, Lieberman HB and Karnitz LM . (2003). J. Biol. Chem., 278, 24428–24437.
St Onge RP, Besley BD, Pelley JL and Davey S . (2003). J. Biol. Chem., 278, 26620–26628.
St Onge RP, Udell CM, Casselman R and Davey S . (1999). Mol. Cell. Biol., 10, 1985–1995.
Tsurimoto T . (1999). Front. Biosci., 4, D849–D858.
Venclovas C and Thelen MP . (2000). Nucleic Acids Res., 28, 2481–2493.
Wang X, Zou L, Zheng H, Wei Q, Elledge SJ and Li L . (2003). Genes Dev., 17, 965–970.
Yoshida K, Komatsu K, Wang HG and Kufe D . (2002). Mol. Cell. Biol., 22, 3292–3300.
Yoshida K, Wang HG, Miki Y and Kufe D . (2003). EMBO J., 22, 1431–1441.
Zhou BB and Elledge SJ . (2000). Nature, 408, 433–439.
Zou L, Cortez D and Elledge SJ . (2002). Genes Dev., 16, 198–208.
Acknowledgements
We thank Michael W Lee for critically reading the paper. This work was supported by grants from the National Institutes of Health (CA90315) to H-GW and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant 15790204) to IH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirai, I., Sasaki, T. & Wang, HG. Human hRad1 but not hRad9 protects hHus1 from ubiquitin–proteasomal degradation. Oncogene 23, 5124–5130 (2004). https://doi.org/10.1038/sj.onc.1207658
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207658
Keywords
This article is cited by
-
Development of Bimolecular Fluorescence Complementation Using Dronpa for Visualization of Protein–Protein Interactions in Cells
Molecular Imaging and Biology (2010)