Abstract
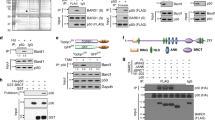
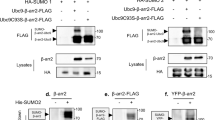
The breast cancer-associated protein, BARD1, colocalizes with BRCA1 in nuclear foci in the S phase and after DNA damage, and the two proteins form a stable heterodimer implicated in DNA repair, protein ubiquitination, and control of mRNA processing. BARD1 has a BRCA1-independent proapoptotic activity; however, little is known about its regulation. Here, we show that BARD1 localization and apoptotic activity are regulated by nuclear–cytoplasmic shuttling. We identified a functional CRM1-dependent nuclear export sequence (NES) near the N-terminal RING domain of BARD1. The NES forms part of the BRCA1 dimerization domain, and coexpression of BRCA1 resulted in masking of the NES and nuclear retention of BARD1. In transient expression assays, BARD1 apoptotic activity was stimulated by nuclear export, and both apoptotic function and nuclear export were markedly reduced by BRCA1. Similar findings were obtained for endogenous BARD1. Silencing BRCA1 expression by siRNA, or disrupting the endogenous BARD1/BRCA1 interaction by peptide competition caused a reduction in BARD1 nuclear localization and foci formation, and increased the level of cytoplasmic BARD1 correlating with increased apoptosis. Our findings suggest that BRCA1/BARD1 heterodimer formation is important for optimal nuclear targeting of BARD1 and its role in DNA repair and cell survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Baer R and Ludwig T . (2002). Curr. Opin. Gen. Dev., 12, 86–91.
Bogerd HP, Fridell RA, Benson RE, Hua J and Cullen BR . (1996). Mol. Cell. Biol., 16, 4207–4214.
Brzovic PS, Meza JE, King MC and Klevit RE . (2001b). J. Biol. Chem., 276, 41399–41406.
Brzovic PS, Rajagopal P, Hoyt DW, King MC and Klevit RE . (2001a). Nat. Struct. Biol., 8, 833–837.
Chen A, Kleiman FE, Manley JL, Ouchi T and Pan ZQ . (2002). J. Biol. Chem., 277, 22085–22092.
Chiba N and Parvin JD . (2001). J. Biol. Chem., 276, 38549–38554.
Chiba N and Parvin JD . (2002). Cancer Res., 62, 4222–4228.
Fabbro M, Rodriguez JA, Baer R and Henderson BR . (2002). J. Biol. Chem., 277, 21315–21324.
Ghimenti CE, Sensi E, Presciuttini S, Brunetti IM, Conte P, Bevilacqua G and Caligo MA . (2002). Genes Chrom. Cancer, 33, 235–242.
Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H and Ohta T . (2001). J. Biol. Chem., 276, 14537–14540.
Henderson BR and Eleftheriou A . (2000). Exp. Cell Res., 256, 1–12.
Irminger-Finger I, Leung W-C, Li J, Dubois-Dauphin M, Harb J, Feki A, Jefford CE, Sorlano JV, Jaconi M, Montesano R and Krause K-H . (2001). Mol. Cell, 8, 1255–1266.
Irminger-Finger I, Soriano JV, Vaudan G, Montesano R and Sappino AP . (1998). J. Cell. Biol., 143, 1329–1339.
Jin Y, Xu XL, Yang M-CW, Wei F, Ayi T-C, Bowcock AM and Baer R . (1997). Proc. Natl. Acad. Sci. USA, 94, 12075–12080.
Joukov V, Chen J, Fox EA, Green JBA and Livingston DM . (2001). Proc. Natl. Acad. Sci. USA, 90, 12078–12083.
Kleiman FE and Manley JL . (1999). Science, 285, 1576–1579.
Kleiman FE and Manley JL . (2001). Cell, 104, 743–753.
Lorick KL, Jensen JP, Fang S, Ong AM and Hatakeyama S . (1999). Proc. Natl. Acad. Sci. USA, 96, 11354–11369.
McCarthy EE, Celebi JT, Baer R and Ludwig T . (2003). Mol. Cell. Biol., 23, 5056–5063.
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Ahrshman K, Tavigian S, Liu Q, Cochran C, Bennett LM, Ding W, Bell R, Rosenthal J, Hussey C, Tran T, McClure M, Frye C, Hattier T, Phelps R, Haugen-Strano A, Katcher H, Yakumo K, Gholami Z, Shaffer D, Stone S, Bayer S, Wray C, Bogden R, Dayananth P, Ward J, Tonin P, Narod S, Bristow PK, Norris FH, Helvering L, Morrison P, Rosteck P, Lai M, Barrett JC, Lewis C, Neuhausen S, Cannon-Albright L, Goldgar D, Wiseman R, Kamb A and Skolnick MHA . (1994). Science, 266, 66–71.
Rodriguez JA and Henderson BR . (2000). J. Biol. Chem., 275, 38589–38596.
Ruffner H, Joazeiro CAP, Hemmati D, Hunter T and Verma IM . (2001). Proc. Natl. Acad. Sci. USA, 98, 5134–5139.
Scully R, Chen J, Ochs RL, Keegan K, Hoekstra M, Feunteun J and Livingston DM . (1997). Cell, 90, 425–435.
Spahn L, Petermann R, Siligan C, Schmid JA, Aryee DNT and Kovar H . (2002). Cancer Res., 62, 4583–4587.
Sui G, Soohoo C, Affar EB, Gay F, Shi Y, Forrester WC and Shi Y . (2002). Proc. Natl. Acad. Sci. USA, 99, 5515–5520.
Thai TH, Du F, Tsan JT, Jin Y, Phung A, Spillman MA, Massa HF, Muller CY, Ashfaq R, Mathis J, Miller DS, Trask BJ, Baer R and Bowcock AM . (1998). Hum. Mol. Genet., 7, 195–202.
Wu LC, Wang ZW, Tsan JT, Spillman MA, Phung A, Xu XL, Yang MC, Hwang LY, Bowcock AM and Baer R . (1996). Nat. Genet, 14, 430–440.
Xu X, Qiao W, Linke SP, Cao L, Li WM, Furth PA, Harris CC and Deng CX . (2001). Nat. Genet., 28, 266–271.
Acknowledgements
We thank J Holt and R Baer for supplying cDNAs and antibodies. We are grateful to R Kefford and T Cunningham for support at the Westmead Millennium Institute. This work was partly supported by a grant from the National Health and Medical Research Council (NHMRC) of Australia. BRH was supported by an NHMRC Senior Research Fellowship. SS was supported by an Erwin Schroedinger post-doctoral fellowship from the FWF of Austria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodriguez, J., Schüchner, S., Au, W. et al. Nuclear–cytoplasmic shuttling of BARD1 contributes to its proapoptotic activity and is regulated by dimerization with BRCA1. Oncogene 23, 1809–1820 (2004). https://doi.org/10.1038/sj.onc.1207302
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207302
Keywords
This article is cited by
-
A synergetic effect of BARD1 mutations on tumorigenesis
Nature Communications (2021)
-
BRCA1 and BARD1 colocalize mainly in the cytoplasm of breast cancer tumors, and their isoforms show differential expression
Breast Cancer Research and Treatment (2015)
-
Cancer predisposing BARD1 mutations in breast–ovarian cancer families
Breast Cancer Research and Treatment (2012)
-
Is there more to BARD1 than BRCA1?
Nature Reviews Cancer (2006)
-
Subcellular localization and nucleocytoplasmic transport of the chromosomal passenger proteins before nuclear envelope breakdown
Oncogene (2006)