Abstract
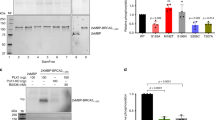
The breast cancer susceptibility protein, BRCA2, preserves chromosomal stability through roles in the repair of DNA double-strand breaks, and possibly, cell division. Post-translational modifications that may coordinate these functions remain poorly characterized. Here, we report that BRCA2 is a substrate for the mitotic Polo-like kinase, Plk1. BRCA2 undergoes phosphorylation in cells synchronously passing through the G2/M phases of cell cycle, when Plk1 expression and activity are maximal. Depletion of Plk1 by RNA interference suppresses BRCA2 modification. BRCA2 and Plk1 interact with one another in cell lysates, through a conserved region in BRCA2, which spans the eight BRC repeat motifs essential for its function in DNA repair. Within this region, residues positioned between BRC repeats – but not the repeat motifs themselves – are phosphorylated by Plk1. Interestingly, Plk1-mediated modification of BRCA2 during the G2/M phases is inhibited by treatment with the radiomimetic agent, adriamycin. Thus, our findings define a regulatory circuit for BRCA2 phosphorylation by Plk1 that is responsive to DNA damage as well as mitotic progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Abrieu A, Brassac T, Galas S, Fisher D, Labbe JC and Doree M . (1998). J. Cell. Sci., 111 (Part 12), 1751–1757.
Alexandru G, Uhlmann F, Mechtler K, Poupart MA and Nasmyth K . (2001). Cell, 105, 459–472.
Baumann P, Benson FE and West SC . (1996). Cell, 87, 757–766.
Bertwistle D and Ashworth A . (1998). Curr. Opin. Genet. Dev., 8, 14–20.
Bork P, Blomberg N and Nilges M . (1996). Nat. Genet., 13, 22–23.
Chen PL, Chen CF, Chen Y, Xiao J, Sharp ZD and Lee WH . (1998a). Proc. Natl. Acad. Sci. USA, 95, 5287–5292.
Chen J, Silver DP, Walpita D, Cantor SB, Gazdar AF, Tomlinson G, Couch FJ, Weber BL, Ashley T, Livingston DM and Scully R . (1998b). Mol. Cell, 2, 317–328.
Connor F, Bertwistle D, Mee PJ, Ross GM, Swift S, Grigorieva E, Tybulewicz VL and Ashworth A . (1997). Nat. Genet., 17, 423–430.
Davies AA, Masson JY, Mcllwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR and West SC . (2001). Mol. Cell, 7, 273–282.
Glover DM, Hagan IM and Tavares AA . (1998). Genes Dev., 12, 3777–3787.
Hamanaka R, Smith MR, O'Connor PM, Maloid S, Mihalic K, Spivak JL, Longo DL and Ferris DK . (1995). J. Biol. Chem., 270, 21086–21091.
Lee KS, Yuan YL, Kuriyama R and Erikson RL . (1995). Mol. Cell. Biol., 15, 7143–7151.
Losada A, Hirano M and Hirano T . (2002). Genes Dev., 16, 3004–3016.
Marmorstein LY, Kinev AV, Chan GK, Bochar DA, Beniya H, Epstein JA, Yen TJ and Shiekhattar R . (2001). Cell, 104, 247–257.
Milner J, Fuks F, Hughes-Davies L and Kouzarides T . (2000). Oncogene, 19, 4441–4445.
Nigg EA . (1998). Curr. Opin. Cell. Biol., 10, 776–783.
Patel KJ, Yu VP, Lee H, Corcoran A, Thistlethwaite FC, Evans MJ, Colledge WH, Friedman LS, Ponder BA and Venkitaraman AR . (1998). Mol. Cell, 1, 347–357.
Pellegrini L, Yu DS, Lo T, Anand S, Lee M, Blundell TL and Venkitaraman AR . (2002). Nature, 420, 287–293.
Qian YW, Erikson E and Maller JL . (1999). Mol. Cell. Biol., 19, 8625–8632.
Qian YW, Erikson E, Taieb FE and Maller JL . (2001). Mol. Biol. Cell., 12, 1791–1799.
Roshak AK, Capper EA, Imburgia C, Fornwald J, Scott G and Marshall LA . (2000). Cell Signal, 12, 405–411.
Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA and Medema RH . (2000). Nat. Cell. Biol., 2, 672–676.
Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, Nigg EA and Peters JM . (2002). Mol. Cell., 9, 515–525.
Sung P . (1994). Science, 265, 1241–1243.
Suzuki A, de la Pompa JL, Hakem R, Elia A, Yoshida R, Mo R, Nishina H, Chuang T, Wakeham A, Itie A, Koo W, Billia P, Ho A, Fukumoto M, Hui CC and Mak TW . (1997). Genes Dev., 11, 1242–1252.
Takahashi T, Sano B, Nagata T, Kato H, Sugiyama Y, Kunieda K, Kimura M, Okano Y and Saji S . (2003). Cancer Sci., 94, 148–152.
Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R and Miyakawa I . (2001a). Cancer Lett., 169, 41–49.
Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R and Miyakawa I . (2001b). Cancer Lett., 164, 41–49.
Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S and Niho Y . (1999). Int. J. Oncol., 15, 687–692.
Toyoshima-Morimoto F, Taniguchi E and Nishida E . (2002). EMBO Rep., 3, 341–348.
Tutt A, Gabriel A, Bertwistle D, Connor F, Paterson H, Peacock J, Ross G and Ashworth A . (1999). Curr. Biol., 9, 1107–1110.
Uchiumi T, Longo DL and Ferris DK . (1997). J. Biol. Chem., 272, 9166–9174.
van Vugt MA, Smits VA, Klompmaker R and Medema RH . (2001). J. Biol. Chem., 276, 41656–41660.
Venkitaraman AR . (2000). Philos. Trans. Roy. Soc. London B Biol. Sci., 355, 191–198.
Venkitaraman AR . (2002). Cell, 108, 171–182.
Weitzer S and Uhlmann F . (2002). Dev. Cell, 2, 381–382.
Wolf G, Elez R, Doermer A, Holtrich U, Ackermann H, Stutte HJ, Altmannsberger HM, Rubsamen-Waigmann H and Strebhardt K . (1997). Oncogene, 14, 543–549.
Wolf G, Hildenbrand R, Schwar C, Grobholz R, Kaufmann M, Stutte HJ, Strebhardt K and Bleyl U . (2000). Pathol. Res. Pract., 196, 753–759.
Wong AK, Pero R, Ormonde PA, Tavtigian SV and Bartel PL . (1997). J. Biol. Chem., 272, 31941–31944.
Wu K, Jiang SW and Couch FJ . (2003). J. Biol. Chem., 278, 15652–15656.
Yu VP, Koehler M, Steinlein C, Schmid M, Hanakahi LA, van Gool AJ, West SC and Venkitaraman AR . (2000). Genes Dev., 14, 1400–1406.
Acknowledgements
This work was supported by grants to ARV from Cancer Research UK and the Medical Research Council. We thank Shubha Anand and Linda Ko Ferrigno for critical comments on this manuscript, and to members of our laboratory for advice and assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Note While this paper was in preparation, an electronic posting in advance of publication from Lee and colleagues (Journal of Biological Chemistry web-site) reports that BRCA2 is phosphorylated on Ser193 during mitosis by Plk1. Consistent with our findings, this residue lies within the fragment, B2-1, whose modification by Plk1 we note here. Our work extends the findings of Lee and colleagues by describing: (1) an in vitro physical association between Plk1 and BRCA2; (2) the phosphorylation of residues between but not within the BRC repeat motifs in exon 11; and (3) the regulation of Plk1-dependent BRCA2 phosphorylation by DNA damage as well as mitotic progression.
Rights and permissions
About this article
Cite this article
Lee, M., Daniels, M. & Venkitaraman, A. Phosphorylation of BRCA2 by the Polo-like kinase Plk1 is regulated by DNA damage and mitotic progression. Oncogene 23, 865–872 (2004). https://doi.org/10.1038/sj.onc.1207223
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207223
Keywords
This article is cited by
-
Proper chromosome alignment depends on BRCA2 phosphorylation by PLK1
Nature Communications (2020)
-
PALB2 connects BRCA1 and BRCA2 in the G2/M checkpoint response
Oncogene (2019)
-
BRCA2 controls DNA:RNA hybrid level at DSBs by mediating RNase H2 recruitment
Nature Communications (2018)
-
Cyclin D1 promotes BRCA2-Rad51 interaction by restricting cyclin A/B-dependent BRCA2 phosphorylation
Oncogene (2016)
-
Discovery and development of the Polo-like kinase inhibitor volasertib in cancer therapy
Leukemia (2015)