Abstract
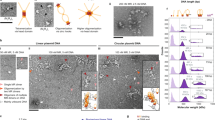
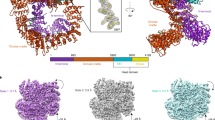
The human tumor suppressor gene ataxia telangiectasia mutated (ATM) encodes a 3056 amino-acid protein kinase that regulates cell cycle checkpoints. ATM is defective in the neurodegenerative and cancer predisposition syndrome ataxia-telangiectasia. ATM protein kinase is activated by DNA damage and responds by phosphorylating downstream effectors involved in cell cycle arrest and DNA repair, such as p53, MDM2, CHEK2, BRCA1 and H2AX. ATM is probably a component of, or in close proximity to, the double-stranded DNA break-sensing machinery. We have observed purified human ATM protein, ATM–DNA and ATM–DNA–avidin bound complexes by single-particle electron microscopy and obtained three-dimensional reconstructions which show that ATM is composed of two main domains comprising a head and an arm. DNA binding to ATM induces a large conformational movement of the arm-like domain. Taken together, these three structures suggest that ATM is capable of interacting with DNA, using its arm to clamp around the double helix.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abraham RT . (2001). Genes Dev., 15, 2177–2196.
Andegeko Y, Moyal L, Mittelman L, Tsarfaty I, Shiloh Y and Rotman G . (2001). J. Biol. Chem., 276, 38224–38230.
Bakkenist CJ and Kastan MB . (2003). Nature, 421, 499–506.
Burma S, Chen BP, Murphy M, Kurimasa A and Chen DJ . (2001). J. Biol. Chem., 276, 42462–42467.
Concannon P . (2002). Nat. Genet., 32, 89–90.
Chiu CY, Cary RB, Chen DJ, Peterson SR and Stewart PL . (1998). J. Mol. Biol., 284, 1075–1081.
Durocher D and Jackson SP . (2001). Curr. Opin. Cell Biol., 13, 225–231.
Ellison V and Stillman B . (2001). Cell, 106, 655–660.
Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M and Leith A . (1996). J. Struct. Biol., 116, 190–199.
Kastan MB and Dae-sik L . (2000). Nat. Rev. Mol. Cell. Biol., 1, 179–186.
Keegan KS, Holtzman DA, Plug AW, Chnstenson ER, Brainerd EE and Flaggs G et al. (1996). Genes Dev., 10, 2423–2437.
Khanna KK, Keating KE, Kozlov S, Scott S, Gatei M, Hobson K, Taya Y, Gabrielli B, Chan D, Lees-Miller SP and Lavin MF . (1998). Nat. Genet., 20, 398–400.
Leuther KK, Hammarsten O, Kornberg R and Chu G . (1999). EMBO J., 18, 1114–1123.
Llorca O, Marco S, Carrascosa JL and Valpuesta JM . (1997). J. Struct. Biol., 118, 31–42.
Llorca O, Martin-Benito J, Grantham J, Ritco-Vonsovici M, Willison KR, Carrascosa JL and Valpuesta JM . (2001). EMBO J., 20, 4065–4075.
Llorca O, McCormack EA, Hynes G, Grantham J, Cordell J, Carrascosa JL, Willison KR, Fernandez JJ and Valpuesta JM . (1999). Nature, 402, 693–696.
Marabini R, Masegosa IM, SanMartin MC, Marco S, Fernandez JJ, De la Fraga LG, Vaquerizo C and Carazo JM . (1996). J. Struct. Biol., 116, 237–240.
Meijers-Heijboer H et al. (2002). Nat. Genet., 31, 55–59.
Perry J and Kleckner N . (2003). Cell, 112, 151–155.
Rhodes N, Gilmer TM and Lansing TJ . (2001). Protein Expression Purification, 22, 462–466.
Rosano C, Arosio P and Bolognesi M . (1999). Biomol. Eng., 16, 5–12.
Rouse J and Jackson SP . (2002). Mol. Cell, 9, 857–869.
Scott S, Bendix R, Chen P, Clark R, Dork T and Lavin MF . (2002). Proc. Natl. Acad. Sci. USA, 99, 925–930.
Smith GCM, Cary RB, Lakin ND, Hann BC, Teo S-H, Chen DJ and Jackson SP . (1999). Proc. Natl. Acad. Sci. USA, 96, 11134–11139.
Spring K, Ahangari F, Scott SP, Waring P, Purdie DM, Chen PC, Hourigan K, Ramsay J, McKinnon PJ, Swift M and Lavin MF . (2002). Nat. Genet., 32, 185–190.
Stroud JC, Lopez-Rodriguez C, Rao A and Chen L . (2002). Nat. Struct. Biol., 9, 90–94.
Suzuki K, Kodama S and Watanabe M . (1999). J. Biol. Chem., 274, 25571–25575.
VanLoop MS, Alexandrov A, Yu X, Cozzarelli MR and Egelman EH . (2002). Curr. Biol, 12, 472–476.
Walker JR, Corpina RA and Goldberg J . (2001). Nature, 412, 607–613.
Yaneva M, Kowalewski T and Lieber MR . (1997). EMBO J., 16, 5098–5112.
Acknowledgements
ATM purified protein was kindly provided by Timothy J Lansing from the Oncology Biology Department, GlaxoSmithkline Research and Development, 5 Moore Drive, Research Triangle Park, NC 27709, USA. From the Institute of Cancer Research, we thank David Robertson, for his help in the initial stages of this work, and the Department of Structural Biology for the use of their computer facilities. We thank Marin van Heel of the Electron Microscopy Unit at Imperial College, London, for use of their microscopes, and John Barber and John Nield of Imperial College for the use of their Leafscan45 scanner (Leaf Systems Inc.). The valuable help of Frank Booy in the use of the electron microscopes at Imperial College is specially acknowledged. Finally, we thank Chris Marshall, David Barford (ICR) and Jasminka Boskovic (CSIC) for careful reading of the manuscript.
This work has been supported by Cancer Research, UK. Oscar Llorca was a Marie Curie Fellow from European Union (Contract number HPMF-CT-2000-00563) at the Institute of Cancer Research till June 2002. OL has also been partially supported by project SAF2002-01715 from the ‘Ministerio de Ciencia y Tecnologia’ of Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Llorca, O., Rivera-Calzada, A., Grantham, J. et al. Electron microscopy and 3D reconstructions reveal that human ATM kinase uses an arm-like domain to clamp around double-stranded DNA. Oncogene 22, 3867–3874 (2003). https://doi.org/10.1038/sj.onc.1206649
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206649
Keywords
This article is cited by
-
The activation mechanisms of master kinases in the DNA damage response
Genome Instability & Disease (2021)
-
Structural basis of homologous recombination
Cellular and Molecular Life Sciences (2020)
-
ATM signalling and cancer
Oncogene (2014)
-
ATM protein kinase: the linchpin of cellular defenses to stress
Cellular and Molecular Life Sciences (2011)
-
Emerging common themes in regulation of PIKKs and PI3Ks
The EMBO Journal (2009)