Abstract
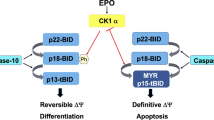
The p53 tumor suppressor gene was found to play a role in the differentiation of several tissue types. We report here that p53-dependent apoptosis plays a role in the final stages of physiological differentiation of normoblasts, resulting in nuclear condensation and expulsion without cell death. Blood samples of healthy newborns, cord blood as well as bone marrow, were analysed for apoptosis by TUNEL and p53 expression by immunostaining. While some samples exhibited simultaneously several distinct patterns of apoptosis, such as perinuclear, diffused nuclear or nuclear apoptotic bodies, others presented a single defined pattern. Overexpression of p53 protein was detected in normoblasts exhibiting either perinuclear or diffused nuclear p53, corresponding to the nuclear apoptotic pattern in the same sample. Similar results were also evident with colonies cultivated for 12–14 days in culture. Differentiated erythroid colonies exhibited overexpression of p53 and positive TUNEL staining only in the normoblasts. We further examined the state of caspase 3/7 and observed a decrease of this activated enzyme during erythroid differentiation in culture. This study suggests a novel role for apoptosis in normoblast differentiation where nuclear degradation occurs with a delay in the actual cell death. A pivotal role for the p53-dependent apoptosis in the erythroid lineage development is implied. However, this apoptotic process is not fully executed because of the exhaustion in caspase 3/7 and thus cells are diverted towards final stages of differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aljurf M, Ma L, Angelucci E, Lucarelli G, Snyder LM, Kiefer CR, Yuan J and Schrier SL . (1996). Blood, 87, 2049–2056.
Almog N and Rotter V . (1997). Biochim. Biopyhs. Acta, 1333, F1–F27.
Aloni-Grinstein R, Zan-Bar I, Alboum I, Goldfinger N and Rotter V . (1993). Oncogene, 8, 3297–3305.
Benito A, Silva M, Grillot D, Nunez G and Fernandez-Luna JL . (1996). Blood, 87, 3837–3843.
Betke K and Kleihauer E . (1967). Nature, 214, 188–189.
Chandrasekaran K, McFarland VW, Simmons DT, Dziadek M, Gurney EG and Mora PT . (1981). Proc. Natl. Acad. Sci. USA, 78, 6953–6957.
Frenkel J, Sherman D, Fein A, Schwartz D, Almog N, Kapon A, Goldfinger N and Rotter V . (1999). Oncogene, 18, 2901–2907.
Kastan M . (1996). BioEssays, 18, 617–619.
Kastan MB, Radin AI, Kuerbitz SJ, Onyekwere O, Wolkow CA, Civin CI, Stone KD, Woo T, Ravindranath Y and Craig RW . (1991). Cancer Res., 51, 4279–4286.
Kelley LL, Green WF, Hicks GG, Bondurant MC, Koury MJ and Ruley HE . (1994). Mol. Cell. Biol., 14, 4183–4192.
Kelley LL, Hicks GG, Hsieh FF, Prasher JM, Green WF, Miller MD, Eide EJ and Ruley HE . (1998). Oncogene, 17, 1119–1130.
Kelley LL, Koury MJ, Bondurant MC, Koury ST, Sawyer ST and Wickerman A . (1993). Blood, 82, 2340–2352.
Kollet O, Aviram R, Chebath J, ben-Hur H, Nagler A, Shultz L, Revel M and Lapidot T . (1999). Blood, 94, 923–931.
Koury MJ and Bondrant MC . (1990). Science, 248, 378–381.
Koury MK, Horne DW, Brown ZA, Pietenpol JA, Blount BC, Ames BN, Hard R and Koury ST . (1997). Blood, 89, 4617–4623.
Law JC, Ritke MK, Yalowich JC, Leder GH and Ferrel R . (1993). Leuk Res., 17, 1045–1050.
Louis JM, McFarland VW, May P and Mora PT . (1988). Biochim. Biophys. Acta, 950, 395–402.
Mahdi T, Alcalay D, Brizard A, Bois M, Millet C, Kitzis A and Tanzer J . (1996). Exp. Hematol., 24, 702–712.
Mora PT, Chandrasekaran K and McFarland VW . (1980). Nature, 288, 722–724.
Morioka K, Tone S, Mukaida M and Takano-Ohmuros H . (1998). Exp. Cell Res., 240, 206–217.
Peller S, Yona R, Kopilova Y, Prokocimer M, Goldfinger N, Uysal A, Karabulut HG, Tukun A, Bokesoy I, Tuncman G and Rotter V . (1998). Genes Chromosomes Cancer, 21, 2–7.
Polyak K, Xia Y, Zweier JL, Kinzler KW and Vogelstein B . (1997). Nature, 389, 300–305.
Rehberger PA, Richter KH, Schwartz D, Goldfinger N, Oskato R, Almog N, Marks F and Rotter V . (1997). Cell Growth Differ., 8, 851–860.
Rogel A, Poplinker M, Webb SG and Oren M . (1985). Mol. Cell Biol., 5, 2851–2855.
Rotter V, Schwartz D, Almon E, Goldfinger N, Kapon A, Meshorer A, Donehower LA and Levine AJ . (1993). Proc. Natl. Acad. Sci. USA, 90, 9075–9079.
Schmid P, Lorenz A, Hameister H and Montenarh M . (1991). Development, 113, 857–865.
Schwartz D, Goldfinger N, Kam Z and Rotter V . (1999). Cell Growth Differ, 10, 665–675.
Schwartz D, Goldfinger N and Rotter V . (1993). Oncogene, 8, 1487–1494.
Silva M, Grillot D, Benito A, Richard C, Nunez G and Fernandez-Luna JL . (1996). Blood, 88, 1576–1582.
Silva M, Richard C, Benito A, Sanz C, Olalla I and Ferandez-Luna JL . (1998). N. Engl. J. Med., 338, 564–571.
Wintrobe . (1999). Wintrobe's Clinical Hematology, 10th edn. Williams andQ4 Wilkins: USA.
Wlodarski P, Wasik M, Ratajczak MZ, Sevignani C, Hoser G, Kawiak J, Gewirtz AM, Calabretta B and Skorski T . (1998). Blood, 91, 2998–3006.
Yonish-Rouach E, Resnitzky K, Lotem J, Sachs L, Kimchi A and Oren M . (1991). Nature, 352, 345–347.
Yuan J, Angelucci E, Lucarelli G, Alijuf M, Snyder LM, Kiefer CR, Ma L and Schrier SL . (1993). Blood, 82, 374–377.
Zauberman A, Lupo A and Oren M . (1995). Oncogenes, 10, 2361–2366.
Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E and Hermine O . (2001). J. Exp. Med., 193, 247–254.
Zeuner A, Eramo A, Peschle C and De Maria R . (1999). Cell Death, 6, 1075–1080.
Acknowledgements
The excellent technical assistance of Mrs Miri Rotschild is acknowledged. This study was supported by a grant from the Israel Cancer Association (SP) and in part by grants from the Israel–USA Binational Science Foundation (BSF) (VR), and the Kadoori Foundation VR. VR is the incumbent of the Norman and Helen Asher Professorial Chair in Cancer Research at the Weizmann Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peller, S., Frenkel, J., Lapidot, T. et al. The onset of p53-dependent apoptosis plays a role in terminal differentiation of human normoblasts. Oncogene 22, 4648–4655 (2003). https://doi.org/10.1038/sj.onc.1206541
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206541
Keywords
This article is cited by
-
Evolutionary conservation of a regulative pathway of erythropoiesis in Poikilothermic vertebrates
Scientific Reports (2022)
-
The notch pathway positively regulates programmed cell death during erythroid differentiation
Leukemia (2007)
-
P53 and Beta-Catenin Activity during Estrogen treatment of Osteoblasts
Cancer Cell International (2005)
-
Vital functions for lethal caspases
Oncogene (2005)
-
Apoptotic mechanisms in the control of erythropoiesis
Leukemia (2004)