Abstract
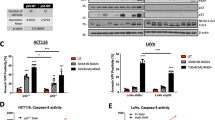
The nonsteroidal anti-inflammatory drugs (NSAIDs) are believed to mediate their anticancer effects by inducing apoptosis but the molecular mechanisms of their apoptotic effects remain largely unknown. Here we report that two different NSAIDs, sulindac sulfide and SC-′236 engage the death receptor 5 (DR5) and mitochondrial pathways to mediate apoptosis in human colon cancer cells. We show that sulindac sulfide and SC-′236-induced apoptosis is coupled with upregulation of DR5, caspase 8 activation and Bid cleavage. Thus, a cross talk appears to exist between the DR5 and mitochondrial pathways during apoptosis induced by these NSAIDs. We further show that sulindac sulfide and SC-′236-induced DR5 upregulation occurs independent of the COX inhibitory effects of these NSAIDs. Using Bax-proficient (Bax+/−) and Bax-deficient (Bax−/−) HCT116 human colon cancer cells, we further demonstrate that Apo2L/TRAIL differentially modulates the apoptotic effects of sulindac sulfide and SC-′236. For example, sulindac sulfide upregulates DR5 in both Bax-deficient and proficient cells, but Apo2L/TRAIL efficiently potentiates sulindac sulfide-induced apoptosis as well as activation of caspase-8, -9 and -3 only in Bax-proficient cells. SC-′236 also upregulates DR5 in both Bax-proficient and Bax-deficient cells but Apo2L/TRAIL potentiates SC-′236-mediated apoptosis and caspases-8 and -3 activation in both Bax-proficient and Bax-deficient cells. Further, in Bax-deficient cells, neither sulindac sulfide nor SC-′236 in combination with Apo2L/TRAIL effectively promotes the release of cytochrome c from mitochondria into cytosol and caspase-9 activation. Collectively, our results suggest that unlike sulindac sulfide, SC-′236 in combination with Apo2L/TRAIL can overcome Bax deficiency to induce apoptosis. These results have important clinical implications in that the tumors harboring Bax mutations are likely to develop resistance to sulindac but not to SC-′236-like NSAIDs. In conclusion, the data presented herein form the basis of future in-depth studies to further explore the utility of Apo2L/TRAIL and NSAIDs, in combination, as a novel cancer preventive/therapeutic strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ahnen DJ . 1998 Eur. J. Surg. 582S: 111–114
Ashkenazi A, Dixit VM . 1999 Curr. Opin. Cell Biol. 11: 255–260
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH . 1999 J. Clin. Invest. 104: 155–162
Burns TF, El-Deiry WS . 2001 J. Biol. Chem. 276: 37879–37886
Chan TA, Morin PJ, Vogelstein B, Kinzler KW . 1998 Proc. Natl. Acad. Sci. USA 95: 681–686
Deng Y, Lin Y, Wu X . 2002 Genes Dev. 16: 33–45
Gross A, McDonnell JM, Korsmeyer SJ . 2000 Genes Dev. 13: 1899–1911
Han Z, Pantazis P, Wyche JH, Kouttab N, Kidd VJ, Hendrickson EA . 2001 J. Biol. Chem. 276: 38748–38754
Huang Y, He Q, Rong R, Hillman MJ, Sheikh MS . 2001 Cancer Res. 61: 6918–6924
Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M . 1993 Nature 363: 558–561
Lancaster T, Silagy C . 1994 Dig. Dis. 12: 170–176
LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Askenazi A . 2002 Nature Med. 8: 274–281
Li H, Zhu H, Xu CJ, Yuan J . 1998 Cell 94: 491–501
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X . 1998 Cell 94: 481–490
Lynch HT, de la Chapelle A . 1999 J. Med. Genet. 36: 801–818
Nicholson DW . 2000 Nature 407: 810–816
Prescott SM, Fitzpatrick FA . 2000 Biochem. Biophys. Act. 1470: M69–M78
Ravi R, Bedi A . 2002 Cancer Res. 62: 1583–1587
Reed JC . 2002 Nature Rev. Drug Dev. 1: 111–121
Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, Perucho M . 1997 Science 275: 967–969
Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME . 1998 Eur. J. Biochem. 254: 439–459
Sheikh MS, Li XS, Chen JC, Shao ZM, Ordonez JV, Fontana JA . 1994 Oncogene 9: 3407–3415
Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace Jr AJ, El-Deiry WS . 1998a Cancer Res. 58: 1593–1598
Sheikh MS, Antinore MJ, Huang Y, Fornace Jr AJ . 1998b Oncogene 17: 2555–2563
Sheikh MS, Fornace Jr AJ . 2000 J. Cell Physiol. 182: 171–181
Shiff SJ, Qiao L, Tsai LL, Rigas B . 1995 J. Clin. Invest. 96: 491–503
Smith ML, Hawcroft G, Hull MA . 2000 Eur. J. Cancer 36: 664–674
Song X, Lin HP, Johnson AJ, Tseng PH, Yang YT, Kulp SK, Chen CS . 2002 J. Natl. Cancer Inst. 94: 585–591
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH . 1999 Nat. Med. 5: 157–163
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ . 2000 Genes Dev. 14: 2060–2071
Williams CS, Mann M, DuBois RN . 1999 Oncogene 18: 7908–7915
Williams CS, Watson AJ, Sheng H, Helou R, Shao J, DuBois RN . 2000 Cancer Res. 60: 6045–6051
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X . 1997 Science 275: 1129–1132
Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B . 2000 Science 290: 989–992
Acknowledgements
We are thankful to Dr Avi Ashkenazi and Genetech for providing Apo2L/TRAIL. We also thank Merck (Rahway, NJ, USA) for supplying sulindac sulfide and Searle/Pharmacia (Chesterfield, MO, USA) for providing SC-′236. This work was supported in part by United States Army Prostate Cancer Research Program grant DAMD 170010722 and by NIH Grant CA89043.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, Q., Luo, X., Huang, Y. et al. Apo2L/TRAIL differentially modulates the apoptotic effects of sulindac and a COX-2 selective non-steroidal anti-inflammatory agent in Bax-deficient cells. Oncogene 21, 6032–6040 (2002). https://doi.org/10.1038/sj.onc.1205897
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205897
Keywords
This article is cited by
-
Myeloid zinc finger 1 mediates sulindac sulfide-induced upregulation of death receptor 5 of human colon cancer cells
Scientific Reports (2014)
-
Celecoxib and a novel COX-2 inhibitor ON09310 upregulate death receptor 5 expression via GADD153/CHOP
Oncogene (2008)
-
Transcriptional upregulation of PUMA modulates endoplasmic reticulum calcium pool depletion-induced apoptosis via Bax activation
Cell Death & Differentiation (2005)
-
Bax deficiency affects caspase-2 activation during ultraviolet radiation-induced apoptosis
Oncogene (2004)
-
Histone deacetylase inhibitors upregulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells
Oncogene (2004)