Abstract
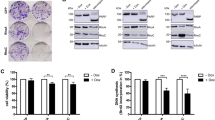
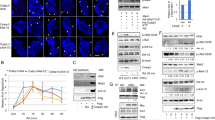
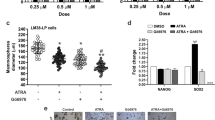
We have previously shown that a retinoic acid receptor (RAR) antagonist BMS453, which does not activate RAR-dependent gene transcription in breast cells, inhibits normal breast cell growth. In this study we have investigated the mechanisms by which this retinoid receptor antagonist inhibits cell growth. Both all trans retinoic acid (atRA) and BMS453 inhibited the proliferation of normal breast cell growth without significantly inducing apoptosis. Both retinoids caused a G1 block in the cell cycle with an increase in the proportion of cells in G0/G1 and a decrease in the proportion of cells in S phase. We then investigated the effects of the retinoids on molecules that regulate the G1 to S transition. These studies demonstrated that both atRA and BMS453 induce Rb hypophosphorylation and decrease CDK2 kinase activity. We then studied the effect of the retinoids on the expression of CDK inhibitors. atRA and BMS453 increased total p21 protein levels and CDK2-bound p21 protein, but did not change CDK4-bound p21. These results suggest that atRA and BMS453 increase p21, decrease CDK2 kinase activity, which in turn leads to hypophosphorylation of Rb and G1 arrest. Because transforming growth factor beta (TGFβ) has been proposed as a mediator of retinoid-induced growth inhibition, we next investigated whether TGFβ mediates the anti-proliferative effect of atRA and BMS453 in normal breast cells. These studies showed that atRA and BMS453 increased total TGFβ activity by 3–5-fold. However, BMS453 increased active TGFβ activity by 33-fold while atRA increased active TGFβ activity by only threefold. These results suggest that BMS453 treatment induces conversion of latent TGFβ to active TGFβ. To investigate whether this increase in active TGFβ mediates the anti-proliferative effects of these retinoids, a TGFβ-blocking antibody was used in an attempt to prevent retinoid-induced growth inhibition. Results from these experiments showed that the anti-TGFβ antibody prevented the inhibition of cell proliferation induced by BMS453, but did not prevent the inhibition of cell proliferation induced by atRA. These results demonstrate that BMS453 inhibits breast cell growth predominantly through the induction of active TGFβ, while atRA inhibits growth through other mechanisms. These results suggest that retinoid analogs that increase active TGFβ may be promising agents for the prevention of breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Abbreviations
- atRA:
-
all trans retinoic acid
- 9cRA:
-
9-cis-retinoic acid
- 13cRA:
-
13 cis retinoic acid
- RAR:
-
retinoic acid receptor
- RXR:
-
retinoic acid X receptor
- ER:
-
estrogen receptor, CDK, cyclin dependent kinase
- TGFβ:
-
transforming growth factor beta
- HMEC:
-
human mammary epithelial cells.
References
Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB . 1994 Analytical Biochem. 216: 276–284
Cassidy J, Liffman M, Lacroix A, Peck G . 1982 Eur. J. Cancer Clin. Oncol. 18: 925–928
Chen JY, Penco S, Ostrowski J, Balague P, Pon M, Starrett J, Reczek P, Chambon P, Gronemeyer H . 1995 EMBO J. 14: 1187–1197
Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ . 1979 Biochem. 18: 5294–5299
Fanjul AN, Piedrafita EJ, Al-Shamma H, Pfahl M . 1998 Cancer Research 58: 4607–4610
Gangemi RM, Costa G, Fulco RA, D'aquino S, Aronadio O . 1996 Ann. NY Acad. Sci. 784: 550–554
Gleizes P-E, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera I, Rifkin D . 1997 Stem Cells 15: 190–197
Glick AB, Flanders KC, Danielpour D, Yuspa SH, Sporn MB . 1989 Cell Regul. 1: 87–97
Hunter T, Pines J . 1994 Cell 79: 573–582
Imai S, Okuno M, Moriwaki H, Muto Y, Murakami K, Shudo K, Suzuki Y, Kojima S . 1997 FEBS Lett. 411: 102–106
Jing Y, Zhang J, Waxman S, Mira-y-Lopez R . 1996 Differentiation 60: 109–117
Kim SJ, Glick A, Sporn MB, Roberts AB . 1989 J. Biol. Chem. 264: 402–408
Kojima S, Rifkin DB . 1993 J. Cell. Physiol. 155: 323–332
Lafyatis R, Lechleider R, Kim SJ, Jakowlew S, Roberts AB, Sporn MB . 1990 J. Biol. Chem. 265: 19128–19136
Lagna G, Hata A, Hemmati-Brvanlou, Massague J . 1996 Nature 383: 832–836
Langenfeld J, Lonardo F, Kiyokawa H, Passalaris T, Ahn M, Rusch V, Dmitrovsky E . 1996 Oncogene 13: 1983–1990
Lee JS, Newman RA, Lippman SM, Huber MH, Minor T, Raber MN, Krakoff IH, Hong WK . 1993 Journal of Clinical Oncology 11: 959–966
Lee PT, Lee MT, Darcy KM, Shudo K, Ip MM . 1995 Endocrinology 136: 1707–1717
Li X, Rishi A, Shao Z, Dawson M, Jong L, Shroot B, Reichert U, Ordonez J, Fontana J . 1996 Cancer Research 56: 5055–5062
Li X-S, Shao Z-M, Sheikh MS, Eiseman JL, Sentz D, Jetten AM, Chen J-C, Dawson MI, Aisner S, Rishi AK, Gutierrez P, Schnapper L, Fontana JA . 1995 Journal of Cellular Physiology 165: 449–458
Lin F, Xiao D, Kolluri S, Zhang X . 2000 Cancer Research 60: 3271–3280
Liu R, Lee M-O, Wang H-G, Li Y, Hashimoto Y, Klaus M, Reed JC, Zhang X-K . 1996 Molecular and Cellular Biology 16: 1138–1149
Liu X, Yue J, Frey RS, Zhu Q, Mulder KM . 1998 Cancer Res. 58: 4752–4757
Miller VA, Rigas JR, Benedetti FM, Verret AL, Tong WP, Kris MG, Gill GM, Loewen GR, Truglia JA, Ulm EH, Warrell Jr RP . 1996 Clinical Cancer Research 2: 471–475
Noma T, Glick AB, Geiser AG, O'Reilly MA, Miller J, Roberts AB, Sporn MB . 1991 Growth Factors 4: 247–255
Nunes J, Kojima S, Rifkin DB . 1996 Cancer Res. 56: 495–499
Pfahl M . 1993 Endocrine Rev. 14: 651–658
Roberts AB, Sporn MB . 1984 The Retinoids. Sporn MB, Roberts AB and Goodman DS (eds). Academic Press: Orlando, FL pp 209–286 443–520
Rocha RL, Hilsenbeck SG, Jackson JG, Lee AV, Figueroa JA, Yee D . 1996 J. Natl. Cancer Inst. 88: 601–606
Seewaldt VL, Johnson BS, Parker MB, Collins SJ, Swisshelm K . 1995 Cell Growth & Differ. 6: 1077–1088
Seewaldt VL, Kim J-H, Caldwell LE, Johnson BS, Swisshelm K, Collins SJ . 1997 Cell Growth & Differ. 8: 631–641
Shao Z-M, Dawson MI, Li XS, Rishi AK, Sheikh MS, Han Q-X, Ordonez JV, Shroot B, Fontana JA . 1995 Oncogene 11: 493–504
Sheikh MS, Shao Z-M, Li X-S, Dawson M, Jetten AM, Wu S, Conley BA, Garcia M, Rochefor H, Fontana JA . 1994 The Journal of Biological Chemistry 269: 21440–21447
Sher C . 1994 Cell 79: 551–555
Sun L, Wu G, Willson JKV, Zborowska E, Yang J, Rajkarunanayake I, Wang J, Gentry LE, Wang X-F, Brattain MG . 1994 J. Biol. Chem. 269: 26449–26455
Sutton LM, Warmith MA, Petros WP, Winter EP . 1997 Cancer Chemother. Pharmacol. 40: 335–341
Teixeira C, Pratt MA . 1997 Mol. Endocrinol. 11: 1191–1202
Toma S, Isnardi L, Riccardi L, Bollag W . 1998a Anticancer Research 18: 935–942
Toma S, Isnardi L, Raffo P, Dastoli G, DeFrancisci E, Riccardi L, Palumbo R, Bollag W . 1997 Int. J. Cancer. 70: 619–627
Toma S, Isnardi L, Raffo P, Riccardi L, Dastoli G, Apfel C, LeMotte P, Bollag W . 1998b Int. J. Cancer 78: 86–94
Yang L, Munoz-Medellin D, Kim H-T, Ostrowski J, Reczek P, Brown PH . 1999 Breast Cancer Research and Treatment 56: 277–291
Yang-Yen HF, Zhang X-K, Graupner G, Tzukerman M, Sakamoto B, Karin M, Pfahl M . 1991 The New Biologist 3: 1206–1219
Zhang Y, Rishi A, Dawson M, Tschang R, Farhana L, Boyanapalli M, Reichert U, Shroot B, Buren EV, Fontana J . 2000 Cancer Research 60: 2025–2032
Zhou Q, Stetler-Stevenson M, Steeg PS . 1997 Oncogene 15: 107–115
Zhu WY, Jones CS, Kiss A, Matsukuma K, Amin S, De Luca LM . 1997 Exp. Cell. Res. 234: 293–299
Acknowledgements
We would like to thank Dr Michael Brattain for providing us with the TGFβ1, 2, 3, TGFβI and II receptor riboplasmids, Dr Doug Yee for providing 36B4 riboplasmid, Dr Rafael Herrera for technical assistance in performing CDK kinase assays, and Dr Marco Gottardis for helpful review of the manuscript. This work was supported by Cancer Research Foundation of America (LM Yang), NIH Grant R01CA78480 (P Brown), and V Foundation for Cancer Research (P Brown).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, L., Ostrowski, J., Reczek, P. et al. The retinoic acid receptor antagonist, BMS453, inhibits normal breast cell growth by inducing active TGFβ and causing cell cycle arrest. Oncogene 20, 8025–8035 (2001). https://doi.org/10.1038/sj.onc.1204911
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204911
Keywords
This article is cited by
-
Advances in Preventive Therapy for Estrogen-Receptor-Negative Breast Cancer
Current Breast Cancer Reports (2014)
-
A clinically relevant bi-cellular murine mammary tumor model as a useful tool for evaluating the effect of retinoic acid signaling on tumor progression
Breast Cancer (2013)
-
SOX9 mediates the retinoic acid-induced HES-1 gene expression in human breast cancer cells
Breast Cancer Research and Treatment (2010)
-
Receptor-selective retinoids inhibit the growth of normal and malignant breast cells by inducing G1 cell cycle blockade
Breast Cancer Research and Treatment (2006)
-
An antagonist of retinoic acid receptors more effectively inhibits growth of human prostate cancer cells than normal prostate epithelium
British Journal of Cancer (2004)