Abstract
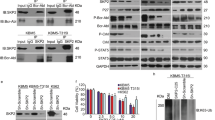
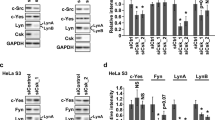
We have previously reported that the Jak2 tyrosine kinase but not Jak1 is tyrosine phosphorylated in the absence of IL-3 in Bcr–Abl positive M3.16 cells, which are rendered IL-3 independent by BCR–ABL gene expression. We have explored the involvement of Jak2 tyrosine phosphorylation in Bcr-Abl oncogenic effects. Our results indicate that Jak2 became tyrosine-phosphorylated in a number of cell lines expressing Bcr–Abl, when maintained in medium lacking IL-3, whereas Bcr–Abl negative cells lacked Jak2 tyrosine phosphorylation. Jak2 was poorly tyrosine-phosphorylated in cells expressing the SH2 deletion mutant of Bcr–Abl compared to either wild-type Bcr–Abl or its SH3 deletion mutant. Moreover, tyrosine phosphorylation of Jak2 by Bcr–Abl was inhibited by the Abl tyrosine kinase inhibitor, STI 571, in a dose-dependent manner. This inhibition of Bcr–Abl kinase by the drug did not interfere with the ability of Jak2 and Bcr–Abl to form a complex. Studies with deletion mutants of Bcr–Abl indicated that the C-terminal domain of Abl within Bcr–Abl was involved in complex formation with Jak2. Similarly, GST–Abl pull-down assays confirmed the strong binding to Jak2 by the C-terminus of Abl. Jak2 peptide substrate studies indicated that the Bcr–Abl and Abl tyrosine kinases specifically phosphorylated Y1007 of Jak2 but only poorly phosphorylated Y1008. Phosphorylation of Y1007 of Jak2 is known to be critical for its tyrosine kinase activation. Tyrosine residue 1007 of Jak2 was phosphorylated in 32Dp210 cells as measured by Western blotting with a phosphotyrosine 1007 sequence-specific antibody. A kinase-inactive Jak2 mutant blocked the colony forming ability of K562 cells. Tumor formation of K562 cells in nude mice was similarly inhibited by this kinase-inactive Jak2 mutant. This inhibition was independent of Stat5 tyrosine phosphorylation. Furthermore, tyrosine-phosphorylated Jak2 was detected in blood cells from CML patients in blast crisis but not in a normal marrow sample. In summary, these findings provide strong evidence that the Jak2 tyrosine kinase is a critical factor in Bcr–Abl malignant transformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Barge RM, de Koning JP, Pouwels K, Dong F, Lowenberg B, Touw IP . 1996 Blood 87: 2148–2153
Carlesso N, Frank DA, Griffin JD . 1996 J. Exp. Med. 811: 811–820
Chai SK, Nichols GL, Rothman P . 1997 J. Immunol. 159: 4720–4728
Danial NN, Losman JA, Lu T, Yip N, Krishnan K, Krolewski J, Goff SP, Wang JYJ, Rothman PB . 1998 Mol. Cell. Biol. 18: 6795–6804
Darnell JE, Kerr IM, Stark GR . 1994 Science 1994: 1415–1421
Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB . 1996 Nature Med. 2: 561–566
Feng J, Witthuhn BA, Matsuda T, Kohlhuber F, Kerr IM, Ihle JN . 1997 Mol. Cell. Biol. 17: 2497–2501
Guo JQ, Hirsch-Ginsberg CF, Xian YM, Stass SA, Champlin RE, Giralt SA, McCredie KB, Campbell ML, Arlinghaus RB . 1993 Hematol. Pathol. 7: 91–106
Horita M, Andreu EJ, Benito A, Arbona C, Sanz C, Benet I, Prosper F, Fernandez-Luna JL . 2000 J. Exp. Med. 191: 977–984
Ilaria RL, Van Etten RA . 1996 J. Biol. Chem. 271: 31704–31710
Lionberger JM, Wilson MB, Smithgall TE . 2000 J. Biol. Chem. 275: 18581–18585
Liu J, Campbell M, Guo JQ, Lu D, Xian YM, Arlinghaus RB . 1993 Oncogene 8: 101–109
Liu J, Wu Y, Arlinghaus RB . 1996 Cancer Res. 56: 5120–5124
Miyazaki T, Takaoka A, Nogueira L, Dikic I, Fuji H, Tsujino S, Mitani Y, Maeda M, Schlessinger J, Taniguchi T . 1998 Genes Dev. 12: 770–775
Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K . 1998 Cell 93: 397–409
Nieborowska-Skorska M, Wasik MA, Slupianek A, Salomoni P, Kitamura T, Calabretta B, Skorski T . 1999 J. Exp. Med. 189: 1229–1242
Parganas E, Wang D, Stravopodis D, Topham DJ, Marine J, Teglund S, Bodner S, Colamonici OR, van Deursen JM, Grosveld G, Ihle JN . 1998 Cell 93: 385–395
Pendergast AM, Quilliam LA, Cripe LD, Bassina CH, Raber KM, Der CJ, Schlessinger L, Gishizky ML . 1993 Cell 75: 175–178
Puil L, Liu J, Gish G, Mbalamu G, Arlinghaus R, Pelicci PG, Pawson T . 1994 EMBO J. 13: 764–773
Sawyers CL . 1999 New Engl. J. Med. 340: 1330–1340
Shuai K, Halpern J, ten Hoeve J, Rao X, Sawyers CL . 1996 Oncogene 13: 247–254
Sillaber C, Gesbert F, Frank DA, Sattler M, Griffin JD . 2000 Blood 95: 2118–2125
Sirard C, Laneuville P, Dick JE . 1994 Blood 15: 1575–1585
Songyang Z, Carraway KL, Eck MJ, Harrison SC, Feldman RA, Mohammad M, Schlessinger J, Hubbard SR, Smith DP, Eng C, Lorenzo MJ, Ponder BAJ, Mayer BJ, Cantley LC . 1995 Nature 373: 536–539
ten Hoeve J, Arlinghaus RB, Guo JQ, Heisterkamp N, Groffen J . 1994 Blood 84: 1731–1736
Ward AC, Touw I, Yoshimura A . 2000 Blood 95: 19–29
Watanabe S, Itoh T, Arai K . 1997 Leukemia 11: 76–78
Wilson-Rawls J, Xie SH, Liu J, Laneuville P, Arlinghaus RB . 1996 Cancer Res. 56: 3426–3430
Wu Y, Ma G, Lu D, Lin F, Xu H-J, Liu J, Arlinghaus RB . 1999 Oncogene 18: 4416–4424
Acknowledgements
This research was supported in part by grants CA16672, CA49639 (RB Arlinghaus) and CA81398 (TE Smithgall) from the National Institute of Health. We thank Novartis Pharmaceuticals, Basel, Switzerland for supplying STI-571. We also thank Drs James Ihle (St. Jude Children's Hospital, Memphis, TN, USA), Tomasz Skorski (Temple University, Philadelphia, PA, USA), Jean Wang (UCSD, La Jolla, CA, USA), Douglas Green (La Jolla Institute for Allergy and Immunology, La Jolla, CA, USA) and Richard Jove (Moffitt Cancer Center, University of South Florida, FL, USA) for important reagents.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, S., Wang, Y., Liu, J. et al. Involvement of Jak2 tyrosine phosphorylation in Bcr–Abl transformation. Oncogene 20, 6188–6195 (2001). https://doi.org/10.1038/sj.onc.1204834
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204834
Keywords
This article is cited by
-
Chronic myeloid leukemia stem cells
Leukemia (2019)
-
Ruxolitinib/nilotinib cotreatment inhibits leukemia-propagating cells in Philadelphia chromosome-positive ALL
Journal of Translational Medicine (2017)
-
Molecular pathogenesis of chronic myeloid leukemia
memo - Magazine of European Medical Oncology (2016)
-
Chronic Myeloid Leukemia in the Era of Tyrosine Kinase Inhibitors: An Evolving Paradigm of Molecularly Targeted Therapy
Molecular Diagnosis & Therapy (2016)
-
miR-101 sensitizes K562 cell line to imatinib through Jak2 downregulation and inhibition of NF-κB target genes
Tumor Biology (2016)