Abstract
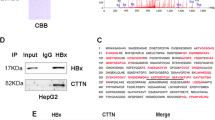
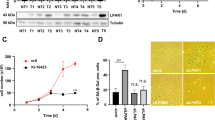
Chronic hepatitis B virus infection is strongly associated with the development of hepatocellular carcinoma (HCC). Epithelial tumors are frequently characterized by loss of cadherin expression or function. Cadherin-dependent adhesion prevents the acquisition of a migratory and invasive phenotype, and loss of its function is itself enough for the progression from adenoma to carcinoma. The HBx protein of hepatitis B virus is thought to contribute to the development of the carcinoma, however, its role in the oncogenic and metastatic processes is far from being fully understood. We report herein the ability of HBx to disrupt intercellular adhesion in three different cell lines stably transfected with an inducible HBx expression vector. The linkage between the actin cytoskeleton and cadherin complex, which is essential for its function, is disrupted in the presence of HBx, as indicated by detergent solubility and immunoprecipitation experiments. In addition, β-catenin was tyrosine phosphorylated in HBx-expressing cells. Inhibition of the src family of tyrosine kinases resulted in the prevention of the disruption of adherens junctions. These results suggest that HBx is able to disrupt intercellular adhesion in a src-dependent manner, and provide a novel mechanism by which HBx may contribute to the development of HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Balsamo J, Leung T, Ernst H, Zanin MKB, Hoffman S, Lilien J . 1996 J. Cell. Biol. 134: 801–813
Baptista M, Kramvis A, Kew MC . 1999 Hepatology 29: 946–953
Behrens J, Vakaet L, Friis R, Winterhager E, Roy FV, Mareel MM, Birchmaier W . 1993 J. Cell. Biol. 120: 757–766
Chisari FV . 2000 Am. J. Pathol. 156: 1118–1132
Christofori G, Semb H . 1999 Trends Biochem. Sci. 24: 73–76
Feitelson MA, Duan L-X . 1997 Am. J. Pathol. 150: 1141–1157
Geiger B, Ayalon O . 1992 Annu. Rev. Cell. Biol. 8: 307–332
Gottlob K, Fulco M, Levrero M, Graessmann A . 1998a J. Biol. Chem. 273: 33347–33353
Gottlob K, Pagano S, Levrero M, Graessmann A . 1998b Cancer Res. 58: 3566–3570
Gumbiner BM . 1996 Cell 84: 345–357
Haviv I, Shamay M, Doitsh G, Shaul Y . 1998 Mol. Cell. Biol. 18: 1562–1569
Hazan RB, Norton L . 1998 J. Biol. Chem. 273: 9078–9084
Kekulé A, Lauer U, Weiss L, Luber B, Hofsschneider P . 1993 Nature 361: 742–745
Kim CM, Koike K, Saito I, Miyamura T, Jay G . 1991 Nature 351: 317–320
Klein NP, Schneider RJ . 1997 Mol. Cell. Biol. 17: 6427–6436
Koike K, Moriya K, Yotsuyanagi H, Lino S, Kurokawa K . 1994 J. Clin. Invest. 94: 44–49
Kozyraki R, Scoazec JY, Flejou JF, D'Errico A, Bedossa P, Terris B, Fiorentino M, Bringuier AF, Grigioni WF, Feldmann G . 1996 Gastroenterology 110: 1137–1149
de La Coste A, Romagnolo B, Billuart P, Renard C-A, Buendia M-A, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C . 1998 Proc. Natl. Acad. Sci. USA 95: 8847–8851
Lara-Pezzi E, Armesilla AL, Majano PL, Redondo JM, López-Cabrera M . 1998 EMBO J. 17: 7066–7077
Lara-Pezzi E, Majano PL, Yáñez-Mó M, Gómez-Gonzalo M, Carretero M, Moreno-Otero R, Sánchez-Madrid F, López-Cabrera M . 2001a J. Hepatol. 34: 409–415
Lara-Pezzi E, Serrador JM, Montoya MC, Zamora D, Yáñez-Mó M, Carretero M, Furthmayr H, Sánchez-Madrid F, López-Cabrera M . 2001b Hepatology in press
Lee TH, Elledge SJ, Butel JS . 1995 J. Virol. 69: 1107–1114
Ludueña MA, Iverson GM, Sussman HH . 1976 J. Cell. Physiol. 91: 119–130
Maguire HF, Hoeffler JP, Siddiqui A . 1991 Science 252: 842–844
Masaki T, Okada M, Shiratori Y, Rengifo W, Matsumoto K, Maeda S, Kato N, Kanai F, Komatsu Y, Nishioka M, Omata M . 1998 Hepatology 27: 1257–1264
Müller T, Choidas A, Reichmann E, UIlrich A . 1999 J. Biol. Chem. 274: 10173–10183
Nhieu JT, Renard C-A, Wei Y, Cherqui D, Zafrani ES, Buendia M-A . 1999 Am. J. Pathol. 155: 703–710
Perl A-K, Wilgenbus P, Dahl U, Semb H, Christofori G . 1998 Nature 392: 190–193
Poussin K, Dienes H, Sirma H, Urban S, Beaugrand M, Franco D, Schirmacher P, Bréchot C, Paterlini-Bréchot P . 1999 Int. J. Cancer 80: 497–505
Provost E, Rimmö DL . 1999 Curr. Opin. Cell. Biol. 11: 567–572
Roche S, Koegl M, Barone MV, Roussel MF, Courtnidge SA . 1995 Mol. Cell. Biol. 15: 1102–1109
Schafer DF, Sorrell MF . 1999 Lancet 353: 1253–1257
Sirma H, Giannini C, Poussin K, Paterlini P, Kremsdorf D, Bréchot C . 1999 Oncogene 18: 4848–4859
Slagle BL, Lee TH, Medina D, Finegold MJ, Butel JS . 1996 Mol. Carcinog 15: 261–269
Slagle BL, Zhou YZ, Birchmeier W, Scorsone KA . 1993 Hepatology 18: 757–762
Tarn C, Bilodeau ML, Hullinger RL, Andrisani OM . 1999 J. Biol. Chem. 274: 2327–2338
Terradillos O, Billet O, Renard C-A, Levy R, Molina T, Briand P, Buendia MA . 1997 Oncogene 14: 395–404
Tlsty TD . 1998 Curr. Opin. Cell. Biol. 10: 647–653
Tsukita S, Tsukita S, Nagafuchi A, Yonemura S . 1992 Curr. Opin. Cell. Biol. 4: 834–839
Ueda H, UIlrich SJ, Gangemi JD, Kappel CA, Ngo L, Feitelson MA, Jay G . 1995 Nat. Genet. 9: 41–47
Vleminckx K, Vakaet L, Mareel M, Fiers W, Roy FV . 1991 Cell 66: 107–119
Yu D-Y, Moon H-B, Son J-K, Jeong S, Yu S-L, Yoon H, Han Y-M, Lee C-S, Park J-S, Lee C-H, Hyun B-H, Murakami S, Lee K-K . 1999 J. Hepatol. 31: 123–132
Zondervan PE, Wink J, Alers JC, IJzermans JN, Schalm SW, de Man RA, van Dekken H . 2000 J. Pathol. 192: 207–215
Acknowledgements
This work was supported by grants FIS 00/0602 from Ministerio de Sanidad y Consumo (to M López-Cabrera) and SAF 99/0034-C01 from Plan Nacional de Salud and 2FD97-0680-C02-02 from Ministerio de Educación y Cultura (to F Sánchez-Madrid). E Lara-Pezzi was supported by a fellowship from the Comunidad Autónoma de Madrid.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lara-Pezzi, E., Roche, S., Andrisani, O. et al. The hepatitis B virus HBx protein induces adherens junction disruption in a src-dependent manner. Oncogene 20, 3323–3331 (2001). https://doi.org/10.1038/sj.onc.1204451
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204451
Keywords
This article is cited by
-
Prediction of hepatocellular carcinoma risk in patients with chronic liver disease from dynamic modular networks
Journal of Translational Medicine (2021)
-
Bioinformatics analysis on multiple Gene Expression Omnibus datasets of the hepatitis B virus infection and its response to the interferon-alpha therapy
BMC Infectious Diseases (2020)
-
Modulation of Wnt signaling pathway by hepatitis B virus
Archives of Virology (2017)
-
Interaction of hepatitis B virus X protein with PARP1 results in inhibition of DNA repair in hepatocellular carcinoma
Oncogene (2016)
-
The unique role of the hepatitis virus B X protein on HEK 293 cell morphology and cellular change
Archives of Virology (2016)