Abstract
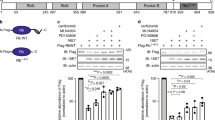
During cellular apoptosis, retinoblastoma protein (RB) is subjected to cleavage near the carboxyl terminus by a caspase-3-like protease. In addition, an heretofore unidentified protease cleaves RB internally, generating fragments of 68 and 48 kDa. Internal cleavage abrogates the ability of RB to associate with E2F. To investigate the mechanism of RB internal cleavage, we developed and employed an in vitro cleavage assay. Incubation of in vitro translated 35S-RB with apoptotic cell extracts led to RB cleavage at the C-terminus, followed by internal cleavage. The caspase peptide inhibitors z-VAD-FMK or z-DEVD-FMK blocked both cleavage events. Rapid C-terminal and internal cleavage were also observed when recombinant caspase-3 was added to 35S-RB. Moreover, when caspase-3 was added to nonapoptotic cell extract, efficient internal cleavage of cellular RB was observed. Caspase-mediated internal cleavage occurred following RB residue aspartate349 in the sequence DSID349. This sequence is consistent with a DXXD recognition motif for caspase-3-like enzymes. Interestingly, we also observed RB internal cleavage in caspase-3-deficient MCF-7 cells, indicating that other caspases are capable of cleaving RB internally. Indeed, caspase-7, a member of the caspase-3 subfamily, was found to cleave 35S-RB at both the carboxyl terminus, and following aspartate349. By contrast, caspases that are not members of the caspase-3 subfamily failed to cleave RB. Taken together, our findings demonstrate that during apoptosis, a caspase-3-like protease is responsible for degradation and functional inactivation of RB by cleaving the protein internally following aspartate349.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Abbreviations
- RB:
-
retinoblastoma protein
- z-VAD-FMK:
-
z-Val-Ala-Asp-fluoromethyl-ketone
- z-DEVD-FMK:
-
z-Asp-Glu-Val-Asp-fluoromethyl-ketone
- DTT:
-
dithiothreitol
- DMSO:
-
dimethylsulfoxide
- PBS:
-
phosphate-buffered saline
- HRP:
-
horseradish peroxidase
- pBKS:
-
Bluescript KS vector
- PAGE:
-
polyacrylamide gel electrophoresis
- VP-16:
-
etoposide
References
Almasan A, Yin Y, Kelly RE, Lee EYHP, Bradley A, Li W, Bertino JR, Wahl GM . 1995 Proc. Natl. Acad. Sci. USA 92: 5436–5440
Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J . 1996 Cell 87: 171
An B, Dou QP . 1996 Cancer Res. 56: 438–442
Bagchi S, Weinmann R, Taychaudhuri P . 1991 Cell 65: 1063–1072
Bandara LR, LaThangue NB . 1991 Nature 351: 494–497
Berry DE, Lu Y, Schmidt B, Fallon PG, O'Connell C, Hu SX, Xu HJ, Blanck G . 1996 Oncogene 12: 1809–1819
Buchovich K, Duffy LA, Harlow E . 1989 Cell 58: 1097–1105
Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR . 1991 Cell 65: 1053–1061
Chen P, Scully P, Shew J, Wang JYJ, Lee WH . 1989 Cell 58: 1193–1198
Chen W, Otterson GA, Lipkowitz S, Khleif SN, Coxon AB, Kaye FJ . 1997 Oncogene 14: 1243–1248
Chittenden T, Livingston DM, Kaelin WG . 1991 Cell 65: 1073–1082
Clarke AR, Maandag ER, van Roon M, van der Lugt NMT, van der Valk M, Hooper ML, Berns A, de Riele H . 1992 Nature 359: 328–330
DeCaprio JA, Ludlow JW, Lynch D, Furukawa Y, Griffin J, Piwnica-Worms H, Huang CM, Livingston DM . 1989 Cell 58: 1085–1095
DeCaprio JA, Furukawa Y, Ajchenbaum F, Griffen J, Livingston DM . 1992 Proc. Natl. Acad. Sci. USA 89: 1795–1798
Dou QP . 1997 Apoptosis 2: 5–18
Dou QP, An B, Antoku K, Johnson DE . 1997 J. Cell Biochem. 64: 586–594
Fattman CL, An B, Dou QP . 1997 J. Cell. Biochem. 67: 399–408
Fattman CL, An B, Sussman L, Dou QP . 1998 Canc. Let. 130: 103–113
Field SJ, Tsai F, Kuo F, Zubiaga AM, Kaelin WG, Livingston DM, Orkin SH, Greenberg ME . 1996 Cell 85: 549–561
Hass-Kogan DA, Kogan SC, Levi D, Dazin P, T'Ang A, Fung YK, Israel, MA . 1995 EMBO J. 14: 461–472
Haupt Y, Rowan S, Oren M . 1995 Oncogene 10: 1563–1571
Huang HJ, Yee JK, Shew JY, Chen PL, Bookstein R, Friedmann T, Lee EY, Lee WH . 1988 Science 242: 1563–1566
Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA . 1992 Nature 359: 295–300
Janicke RU, Walker PA, Lin XY, Porter AG . 1996 EMBO J. 15: 6969–6978
Johnson DG, Schwartz JK, Cress WD, Nevins JR . 1993 Nature 365: 349–352
Kranenburg O, van der Eb AJ, Zantema A . 1996 EMBO J. 15: 46–54
Kurokawa H, Nishio K, Fukumoto H, Tomonari A, Suzuki T, Saijo N . 1999 Oncol. Reports 6: 33–37
Lee EYHP, Chang CY, Hu N, Wang YCJ, Lai CC, Herrup K, Lee WH, Bradley A . 1992 Nature 359: 288–294
McConkey DJ, Goodrich D, Bucana C, Klostergaard J . 1996 Oncogene 13: 1693–1700
Meikranz W, Gisselbrecht S, Tam S, Schlegel R . 1994 Proc. Natl. Acad. Sci. USA 91: 3754–3758
Mihara K, Cao X, Yen A, Chandler S, Driscoll B, Murphree AL, T'Ang A, Fung YK . 1989 Science 246: 1300–1303
Qin X, Livingston DM, Kaelin WG, Adams P . 1994 Proc. Natl. Acad. Sci. USA 91: 10918–10922
Shan B, Farmer AA, Lee WH . 1996 Cell Growth Diff. 7: 689–697
Tan X, Wang JYJ . 1998 Trends in Cell Biol. 8: 116–120
Tan X, Martin SJ, Green DR, Wang JYJ . 1996 J. Biol. Chem. 272: 9613–9616
Thornberry NA, Lazebnik Y . 1998 Science 281: 1312–1316
Wang JYJ . 1997 Curr. Opin. Gen. Devel. 7: 39–45
Weinberg RA . 1991 Science 254: 1138–1146
Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ . 1996 Cell 85: 537–548
Acknowledgements
We thank Wen-Hwa Lee for the generous gift of pLRBRNL plasmid. We also thank Bing An and Keiko Antoku for helpful discussions. This work was supported by National Institutes of Health Grants CA66044 to DE Johnson and AG13300 to QP Dou.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fattman, C., Delach, S., Dou, Q. et al. Sequential two-step cleavage of the retinoblastoma protein by caspase-3/-7 during etoposide-induced apoptosis. Oncogene 20, 2918–2926 (2001). https://doi.org/10.1038/sj.onc.1204414
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204414