Abstract
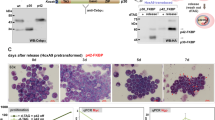
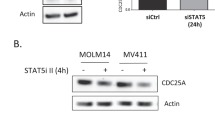
Members of the cdc25 family are protein phosphatases that play pivotal roles in cell cycle progression. Cdc25A has been shown to be a critical regulator of the G1/S transition of mammalian cells and to be a myc-target gene with oncongenic properties. We investigated the regulation of cdc25A during terminal differentiation using myeloblastic leukemia M1 cells, that can be induced to undergo differentiation into macrophages by interleukin-6 (IL-6) treatment. In this report it is shown that cdc25A protein is degraded by the ubiquitin-proteasome machinery in both terminally differentiating and cycling cells. Cdc25A was found to have two major peaks of accumulation during cell cycle progression, one in G1 and the other in S/G2. Evidence was obtained that degradation of cdc25A by the ubiquitin-proteasome machinery in terminally differentiating myeloid cells is accelerated compared to cycling cells. Moreover, deregulated expression of c-myc in M1 cells, which had been previously shown to block terminal differentiation, was also found to block IL-6 induced degradation of cdc25A.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Baldin V, Cans C, Knibiehler M and Ducommun B . 1997 J Biol Chem 272: 32731–32734
Blomberg I and Hoffmann I . 1999 Mol Cell Biol 19: 6183–6194
Ciechanover A . 1994 Cell 79: 13–21
Ciechanover A . 1998 EMBO J 17: 7151–7160
Dick LR, Cruikshank AA, Destree AT, Grenier L, McCormack TA, Melandri FD, Nunes SL, Palombella VJ, Parent LA, Plamodon L and Stein RL . 1997 J Biol Chem 272: 182–188
Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth PK and Dotto GP . 1998 Science 280: 1069–1072
Dixon D, Moyana T and King MJ . 1998 Exp Cell Res 240: 236–243
Draetta G and Eckstein J . 1997 Biochim Biophys Acta 1132: M53–M63
Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ and Schreiber SL . 1995 Science 268: 726–731
Galaktionov K and Beach D . 1991 Cell 67: 1181–1194
Galaktionov K, Lee AK, Eckstein J, Draetta G, Meckler J, Loda M and Beach D . 1995 Science 296: 1575–1577
Galaktionov K, Chem X and Beach D . 1996 Nature 382: 511–517
Hershko A and Ciechanover A . 1992 Ann Rev Biochem 61: 761–807
Hershko A . 1997 Curr Opin Cell Biol 9: 788–799
Hochstrasser M . 1996 Ann Rev Genet 30: 405–439
Hoffman-Liebermann B and Liebermann DA . 1991a Mol Cell Biol 11: 2375–2381
Hoffman-Liebermann B and Liebermann DA . 1991b Oncogene 6: 903–909
Hoffmann I, Clarke P, Marcote MJ, Karsenti E and Draetta G . 1993 EMBO J 13: 53–63
Hoffmann I, Draetta G and Karsenti E . 1994 EMBO J 13: 4302–4310
Honda R, Ohba Y, Nagata A, Okayama H and Yasuda H . 1993 FEBS lett 318: 331–334
Hoyt MA . 1997 Cell 91: 149–151
Iavarone A and Massague J . 1997 Nature 387: 417–422
Jinno S, Suto K, Nagata A, Igarashi M, Kanaoka Y, Nojima H and Okayama H . 1994 EMBO J 13: 1549–1556
Karlsson C, Katich S, Hagting A, Hoffmann I and Pines J . 1999 J Cell Biol 146: 573–584
King RW, Deshaies RJ, Peters M and Kirschner MW . 1996 Science 274: 1652–1659
Krishnaraju K, Hoffman B and Liebermann D . 1998 Blood 92: 1957–1966
Lammer C, Wagerer S, Saffrich R, Mertens D, Ansorge W and Hoffmann I . 1998 J Cell Sci 111: 2445–2453
Laney JD and Hochstrasser M . 1999 Cell 97: 427–430
Lew DJ and Kornbluth S . 1996 Curr Opin Cell Biol 8: 795–804
Morgan DO . 1995 Nature 374: 131–134
Nagata A, Igarashi M, Jinno S, Suto K and Okayama H . 1991 New Biol 3: 959–968
Nefsky B and Beach D . 1996 EMBO J 15: 1301–1312
Norbury C and Nurse P . 1992 Annu Rev Biochem 61: 441–470
Parsons R . 1998 Curr Opin Oncol 10: 88–91
Sadhu K, Reed SI, Richardson H and Russel P . 1990 Proc Natl Acad Sci USA 87: 5139–5143
Selvakumaran M, Liebermann D and Hoffman-Liebermann B . 1993 Blood 81: 2257–2262
Sexl V, Diehl JA, Sherr CJ, Ashmun R, Beach D and Russel MF . 1999 Oncogene 18: 573–582
Sherr CJ and Roberts JM . 1995 Genes Dev 9: 1149–1163
Spinella MJ, Freemantle SJ, Sekula D, Chang JH, Christie AJ and Dmistrovsky E . 1999 J Biol Chem 274: 22013–22018
Strausfeld U, Labbe JC, Fesquet D, Cavadore JK, Picard A, Sadhu K, Russel P and Doree M . 1991 Nature 351: 242–245
Tiefenbrun N, Melamed D, Levy N, Resnitsky D, Hoffmann I, Reed SI and Kimchi A . 1996 Mol Cell Biol 16: 3934–3944
Vigo E, Muller H, Prosperini E, Hateboer G, Cartwright P, Moroni MC and Helin K . 1999 Mol Cell Biol 19: 6379–6395
Acknowledgements
We thank Dr Xavier Grana for critical reading of the manuscript and Dr Dale Haines for advice. This work was supported by National Institute of Health grant 1RO1CA51168 (B Hoffman) and 1RO1CA4316 (D Liebermann).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bernardi, R., Liebermann, D. & Hoffman, B. Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene 19, 2447–2454 (2000). https://doi.org/10.1038/sj.onc.1203564
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203564
Keywords
This article is cited by
-
Identification of VWA5A as a novel biomarker for inhibiting metastasis in breast cancer by machine-learning based protein prioritization
Scientific Reports (2024)
-
Genome-scale screening of deubiquitinase subfamily identifies USP3 as a stabilizer of Cdc25A regulating cell cycle in cancer
Cell Death & Differentiation (2020)
-
The REGγ inhibitor NIP30 increases sensitivity to chemotherapy in p53-deficient tumor cells
Nature Communications (2020)
-
Role and regulation of Cdc25A phosphatase in neuron death induced by NGF deprivation or β-amyloid
Cell Death Discovery (2016)
-
David and Goliath: chemical perturbation of eukaryotes by bacteria
Journal of Industrial Microbiology and Biotechnology (2016)