Abstract
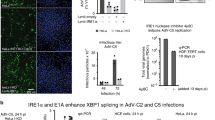
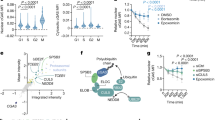
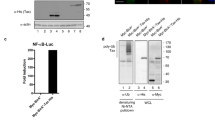
The hepatitis B virus X protein plays an important role in the regulation of viral genome expression and has also been implicated in the development of liver cancer associated with chronic viral infection. Several effects have been attributed to X but their biological relevance remains elusive. One of the confusing issues has been so far the uncertaintys concerning its cellular location. To gain insight into the mechanism(s) how X exerts its effects, we have analysed its subcellular distribution and its dependency on the cell cycle. We used two complementary approaches namely, immunolocalization using a cell line stably expressing X, and characterization of the dynamics of X location in living cells by means of the reporter gene GFP. Our data clearly define the cytosol as the prime location of X, irrespectively of the cell cycle and show in addition the close attachment of a fraction of X to the nuclear membrane. However, X does not associate with any cytoplasmic vesicles and organelles so far tested. In contrast, our study provides strong evidence for the codistribution of X with the cytosolic fraction of proteasomes. In pulse-chase experiments, X decayed with a half-life of less than 30 min and proteasome-inhibitors did not modify its turnover, suggesting that X colocalization with the proteasome does not simply point to its degradation pathway. The proteolytic processing of the p105 precursor of the p50 subunit of the NF-κB transcription factor, which has been shown to be proteasome-dependent, is markedly slow down in the presence of X. These findings suggest that X modulates the processing rate of p105 by acting presumably at the level of the proteasome. Thus, targeting of proteasomes by X might be one of the pathways employed by this viral protein to subvert cellular functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sirma, H., Weil, R., Rosmorduc, O. et al. Cytosol is the prime compartment of hepatitis B virus X protein where it colocalizes with the proteasome. Oncogene 16, 2051–2063 (1998). https://doi.org/10.1038/sj.onc.1201737
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1201737
Keywords
This article is cited by
-
Hepatitis B virus X protein in liver tumor microenvironment
Tumor Biology (2016)
-
Epigenetic repression of E-cadherin expression by hepatitis B virus x antigen in liver cancer
Oncogene (2012)
-
Viral hepatocarcinogenesis
Oncogene (2010)
-
RNAi for Treating Hepatitis B Viral Infection
Pharmaceutical Research (2008)
-
Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis
Oncogene (2006)