Abstract
OBJECTIVE: To compare insulin sensitivity (Si) from a frequently sampled intravenous glucose tolerance test (FSIGT) and subsequent minimal model analyses with surrogate measures of insulin sensitivity and resistance and to compare features of the metabolic syndrome between Caucasians and Indian Asians living in the UK.
SUBJECTS: In all, 27 healthy male volunteers (14 UK Caucasians and 13 UK Indian Asians), with a mean age of 51.2±1.5 y, BMI of 25.8±0.6 kg/m2 and Si of 2.85±0.37.
MEASUREMENTS: Si was determined from an FSIGT with subsequent minimal model analysis. The concentrations of insulin, glucose and nonesterified fatty acids (NEFA) were analysed in fasting plasma and used to calculate surrogate measure of insulin sensitivity (quantitative insulin sensitivity check index (QUICKI), revised QUICKI) and resistance (homeostasis for insulin resistance (HOMA IR), fasting insulin resistance index (FIRI), Bennetts index, fasting insulin, insulin-to-glucose ratio). Plasma concentrations of triacylglycerol (TAG), total cholesterol, high density cholesterol, (HDL-C) and low density cholesterol, (LDL-C) were also measured in the fasted state. Anthropometric measurements were conducted to determine body-fat distribution.
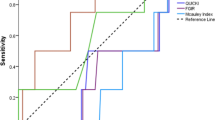
RESULTS: Correlation analysis identified the strongest relationship between Si and the revised QUICKI (r=0.67; P=0.000). Significant associations were also observed between Si and QUICKI (r=0.51; P=0.007), HOMA IR (r=−0.50; P=0.009), FIRI and fasting insulin. The Indian Asian group had lower HDL-C (P=0.001), a higher waist–hip ratio (P=0.01) and were significantly less insulin sensitive (Si) than the Caucasian group (P=0.02).
CONCLUSION: The revised QUICKI demonstrated a statistically strong relationship with the minimal model. However, it was unable to differentiate between insulin-sensitive and -resistant groups in this study. Future larger studies in population groups with varying degrees of insulin sensitivity are recommended to investigate the general applicability of the revised QUICKI surrogate technique.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wallace TM, Matthews DR . The assessment of insulin resistance in man. Diabet Med 2002; 19: 527–534.
Stern MP . Do non insulin dependant diabetes mellitus and cardiovascular disease share common antecedents? Annal Intern Med 1996; 124: 110–116.
Reaven GM . Role of insulin resistance in human disease. Diabetes 1988; 37: 1595–1607.
Zoratti R, Godsland I, Chaturvedi N, Crook D, Stevenson JC, McKeigue P . Relation of plasma lipids to insulin resistance, nonesterified fatty acid levels and body fat in men from three different ethnic groups: relevance to variation in risk of diabetes and coronary heart disease. Metabolism 2000; 49: 245–252.
Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE . Body composition, visceral fat, leptin and insulin resistance in Asian Indian men. J Clin Endocrinol Metab 1998; 84: 137–144.
Chambers JC, Eda S, Bassett P, Karim Y, Thompson SG, Gallimore R, Pepys MB, Kooner JS . C-reactive protein, insulin resistance, central obesity and coronary heart disease risk in Indian Asians from the United Kingdom compared with European Whites. Circulation 2001; 104: 145–150.
Kulkarni K, Markovitz J, Nanda NC, Segrest JP . Increased prevalence of smaller and denser LDL particles in Asian Indians. Arterioscler Thromb Vasc Biol 1999; 19: 2749–2755.
British Heart Foundation. Coronary heart disease statistics. British Heart Foundation: London; 2002.
McKeigue PM, Miller GJ, Marmot MG . Coronary heart disease in South Asians overseas: A review. J Clin Epidemiol 1989; 42: 597–609.
DeFronzo RA, Toin JD, Andres R . Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: 214–223.
Bergman RN . Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 1989; 38: 1512–1527.
Saad MF, Anderson RL, Laws A, Watanbe RM, Kades WW, Chen I, Evan-Sands R, Pei D, Savage PJ, Bergman RN . A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Diabetes 1994; 43: 1114–1121.
Matthews DR, Hosker JP, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Bonora E . Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 2000; 23: 57–63.
Fukushima M, Taniguchi A, Sakai M, Doi K, Nagata I, Nagasaka S, Tokuyama K, Nakai Y . Assessment of insulin sensitivity from a single sample. Diabetes Care 2000; 23: 1434–1435.
Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, Cram KB, Hutchinson RG . Relation of carotid wall thickness to diabetes mellitus, fasting glucose and insulin, body size and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke 1994; 25: 66–73.
Anderson RL, Hamman RF, Savage PJ, Saad MF, Laws A, Kades WW, Sands RE, Cefalu W . Exploration of simple insulin sensitivity measures derived from frequently sampled intravenous glucose tolerance (FSIGT) tests. Am J Epidemiol 1995; 142: 724–732.
Caro J . Insulin resistance in obese and nonobese man. J Clin Endocrinol Metab 1991; 73: 691–695.
McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW . Diagnosing insulin resistance in the general population. Diabetes Care 2001; 24: 460–464.
Ikeda Y, Suehiro T, Nakamura T, Kumon Y, Hashimoto K . Clinical significance of the insulin resistance index as assessed by the homeostasis model assessment. Endocr J 2001; 48: 81–86.
Laasko M . How good a marker is insulin level for insulin resistance. Am J Epidemiol 1993; 137: 959–964.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ . Quantitative insulin sensitivity check index: a simple accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000; 85: 2402–2410.
Perseghin G, Caumo A, Caloni M, Testolin G, Luzi L . Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in nonobese individuals. J Clin Endocrinol Metab 2001; 86: 4776–4781.
Bergman RN, Finegood DT, Ader M . Assessment of insulin sensitivity in vivo. Endocr Rev 1985; 6: 45–86.
Pacini G, Bergman RN . MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsitivity from the frequently sampled intravenous glucose tolerance test. Comput Meth Prog Biomed 1986; 23: 113–122.
Haffner SM, D’Agostino R, Saad MF, Rewers M, Mykkanen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE, Bergman RN . Increased insulin resistance and insulin secretion in non diabetic African-Americans and Hispanics compared with non-Hispanic Whites: the insulin resistance atherosclerosis study. Diabetes 1996; 45: 742–748.
Durnin JV, Wormsley J . Body fat assessed from total body density and its estimation from skinfold thickness measurements on 481 mean and women aged 16 to 72 years. Br J Nutr 1974; 32: 77–97.
Friedewald WT, Levy RI . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Haffner SM, Miettinen H, Stern MP . The homeostasis model in the San Antonio heart study. Diabetes Care 1997; 20: 1087–1092.
Frayn KN . Non esterified fatty acid metabolism and postprandial lipaemia. Atherosclerosis 1998; 141: S41–S46.
Frayn KN, Williams CM, Arner P . Are increased plasma non esterified fatty acid concentrations a risk marker for coronary heart disease and other chronic diseases? Clin Sci 1996; 90: 243–253.
Bhatnager D, Anand IS, Durrington PN, Patel DJ, Wander GS, Mackmess MI, Creed F, Tomenson B, Chandrashekhar Y, Winterbotham M, Britt RP, Keil JE, Sutton GC . Coronary risk factors on people from the Indian subcontinent living in west London and their siblings in India. Lancet 1995; 345: 405–409.
McKeigue PM, Shah B, Marmot MG . Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 1991; 337: 382–386.
Lovegrove JA, Brady LM, Lesauvage SVM, Lovegrove SS, Minihane AM, Williams CM . Lack of association between central adiposity and lipaemia in UK Sikh men. Int J Obesity 27: 1373–1382.
Frost GS, Leogh BE, Smith D, Leeds AR, Dornhurst A . Reduced adipocyte insulin sensitivity in Caucasian and Asian subjects with coronary heart disease. Diabet Med 1998; 15: 1003–1009.
Acknowledgements
We thank the Food Standards Agency (FSA) who provided funding for this research. We also thank Dr John Wright who cannulated and infused all the volunteers with glucose and insulin on the insulin sensitivity study days and Kangmei Ren who helped with the minimal model analysis. Finally, we thank the volunteers who gave up their time to participate in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brady, L., Gower, B., Lovegrove, S. et al. Revised QUICKI provides a strong surrogate estimate of insulin sensitivity when compared with the minimal model. Int J Obes 28, 222–227 (2004). https://doi.org/10.1038/sj.ijo.0802547
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802547
Keywords
This article is cited by
-
When thought suppression backfires: its moderator effect on eating psychopathology
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2015)
-
A preliminary investigation of sex differences and the mediational role of food thought suppression in the relationship between stress and weight cycling
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2010)
-
Fasting‐based Estimates of Insulin Sensitivity in Overweight and Obesity: A Critical Appraisal
Obesity (2006)
-
Lack of association between central adiposity and lipaemia in UK Sikh men
International Journal of Obesity (2003)