Abstract
OBJECTIVE: Several studies have implicated increased sympathetic tone as a contributing factor to the hyperglycemia and hyperglucagonemia of ob/ob mice. However, the responsiveness of plasma glucose, insulin and glucagon to circulating norepinephrine (NE) in ob/ob vs normal lean mice has never been described. Therefore, the present study investigated the effect of a 15 min intravenous NE infusion (1 pmol/min/g) on plasma glucose, insulin and glucagon in anesthetized lean, ob/ob, ob/ob-concurrent yohimbine (α2 antagonist) treated, and ob/ob-chronically sympatholytic dopamine agonist treated (for 14 days prior to infusion) mice. In an effort to gain insight into a possible relation between norepinephrine, hyperglucagonemia and hyperinsulinemia in ob/ob mice, this study also examined the isolated islet responses to NE and glucagon in lean, ob/ob and ob/ob-sympatholytic dopamine agonist treated mice.
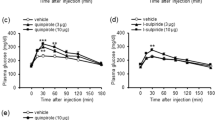
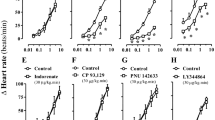
RESULTS: Basal humoral values of glucose, insulin and glucagon were all elevated in ob/ob vs lean mice (by 63, 1900 and 63%, respectively, P<0.01). However, NE infusion further increased levels of glucose, insulin and glucagon in ob/ob (by 80, 90 and 60%, respectively, P<0.05) but not in lean mice (between group difference for all parameters P<0.05). Acute concurrent yohimbine treatment as well as chronic prior sympatholytic dopamine agonist treatment (bromocriptine plus SKF38393) simultaneously strongly aborgated or abolished all these humoral hypersensitivity responses to intravenous NE in ob/ob mice (P<0.05). Clamping the plasma glucose level in untreated ob/ob mice at a high level (30 mM) established by NE infusion did not significantly alter the plasma insulin level, suggesting that some other influence of NE was responsible for this insulin effect. Direct NE administration at 1 μM to islets from lean and ob/ob mice inhibited 15 mM glucose-stimulated insulin secretion in both groups, but at 0.1 μM it was inhibitory only in islets from ob/ob mice. However, glucagon (10 nM) increased 15 mM glucose-stimulated insulin secretion in ob/ob (by 170%, P<0.05) but not lean mice (between group difference P<0.05).
CONCLUSION: These findings suggest that hypersensitivity to circulating NE may potentiate hyperglycemia and hyperglucagonemia in ob/ob mice, and the subsequent hyperglucagonemia coupled with increased islet β-cell insulin secretory responsiveness to glucagon in ob/ob mice may support hyperinsulinemia, thus explaining the increased plasma insulin level response to intravenous NE in these animals. These findings further support a role for increased peripheral noradrenergic activities in the development and maintenance of the hyperglycemic, hyperglucagonemic and hyperinsulinemic state, characteristic of type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Thurlby PL, Trayhurn P . The role of thermoregulatory thermogenesis in the development of obesity in genetically-obese (ob/ob) mice pair-fed with lean siblings Br J Nutr 1979 42: 377–385.
Young JB, Landsberg L . Diminished sympathetic nervous system activity in genetically obese (ob/ob) mouse Am J Physiol 1983 245: E148–E154.
Scarpace PJ, Matheny M . Leptin induction of UCP1 gene expression is dependent on sympathetic innervation Am J Physiol 1998 275: E259–E264.
Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI . Receptor-mediated regional sympathetic nerve activation by leptin J Clin Invest 1997 100: 270–278.
Jacob RJ, Dziura J, Medwick MB, Leone P, Caprio S, During M, Shulman GI, Sherwin RS . The effect of leptin is enhanced by microinjection into the ventromedial hypothalamus Diabetes 1997 46: 150–152.
Oltmans GA . Norepinephrine and dopamine levels in hypothalamic nuclei of the genetically obese mouse (ob/ob) Brain Res 1983 273: 369–373.
Lorden JF, Oltmans GA, Margules DL . Central catecholamine levels in genetically obese mice (ob/ob and db/db) Brain Res 1975 96: 390–394.
Kraszewski K, Cincotta AH . Increased responsiveness of ventromedial hypothalamic neurons to norepinephrine in obese versus lean mice: relation to the metabolic syndrome Int J Mol Med 2000 5: 349–355.
Boundy VA, Cincotta AH . Hypothalamic adrenergic receptor changes in the metabolic syndrome of genetically obese (ob/ob) mice Am J Physiol 2000 279: R505–R514.
Amir S . Intra-ventromedial hypothalamic injection of glutamate stimulates brown adipose tissue thermogenesis in the rat Brain Res 1990 511: 341–344.
Yoshimatsu H, Egawa M, Bray GA . Sympathetic nerve activity after discrete hypothalamic injections of L-glutamate Brain Res 1993 601: 121–128.
Luo S, Luo J, Hodge S et al. Increased daily turnover of noradrenaline and serotonin in ventral medial hypothalamus (VMH) of obese versus lean Zucker rats assessed by in vivo microdialysis (Abstract) Neuroscience 1996 22: 605.
Luo S, Luo J, Cincotta AH . Bromocriptine reduces obesity, glucose intolerance and extracellular monoamine metabolite levels in the ventromedial hypothalamus of Syrian hamsters Neuroendocrinology 1998 68: 1–10.
Jones AP, Pothos EN, Rada P, Olster DH, Hoebel BG . Maternal hormonal manipulations in rats cause obesity and increase medial hypothalamic norepinephrine release in male offspring Brain Res Dev Brain Res 1995 88: 127–131.
Garris DR . Age- and diabetes-associated alterations in regional brain norepinephrine concentrations and adrenergic receptor populations in C57BL/KsJ mice Devl Brain Res 1990 51: 161–166.
Steffens AB, Damsma G, van der Gugten J, Luiten PG . Circulating free fatty acids, insulin and glucose during chemical stimulation of hypothalamus in rats Am J Physiol 1984 247: E765–E771.
Steffens AB, Scheurink AJ, Luiten PG, Bohus B . Hypothalamic food intake regulating areas are involved in the homeostasis of blood glucose and plasma FFA levels Physiol Behav 1988 44: 581–589.
Shimazu T . Neuronal regulation of hepatic glucose metabolism in mammals Diabetes/Metab Rev 1987 3: 185–206.
Shimazu T . Central nervous system regulation of liver and adipose tissue metabolism Diabetologia 1981 20: 343–356.
Porte DJ . Beta-cells in type II diabetes mellitus Diabetes 1991 40: 166–180.
Unger RH . Diabetes and the alpha cell Diabetes 1976 25: 136–151.
Samols E, Bonner-Weir S, Weir GC . Intra-islet insulin-glucagon-somatostatin relationships Clin Endocrinol Metab 1986 15: 33–58.
Liang Y, Lubkin M, Sheng H, Scislowski PW, Cincotta AH . Dopamine agonist treatment ameliorates hyperglycemia, hyperlipidemia, and the elevated basal insulin release from islets of ob/ob mice Biochim Biophys Acta 1998 1405: 1–13.
Liang Y, Luo S, Joslin J et al. Chronic infusion of NE into the VMH causes dysregulation of insulin and glucagon release in normal rat. (Abstract) Diabetes 1999 48 (Suppl 1): A237.
Liang Y, Luo S, Cincotta AH . Long-term infusion of norepinephrine plus serotonin into the ventromedial hypothalamus impairs pancreatic islet function Metabolism 1999 48: 1287–1289.
Luo S, Luo J, Cincotta AH . Chronic ventromedial hypothalamic infusion of norepinephrine and serotonin promotes insulin resistance and glucose intolerance Neuroendocrinology 1999 70: 460–465.
Cincotta AH, Luo S, Zhang Y, Liang Y, Bina KG, Jetton TL, Scislowski PW . Chronic infusion of norepinephrine into the VMH of normal rats induces the obese-glucose intolerant state Am J Physiol Regul Integr Comp Physiol 2000 278: R435–444.
Bina KG, Cincotta AH . Dopaminergic agonists normalize elevated hypothalamic NPY and CRF, body weight gain, and hyperglycemia in ob/ob mice Neuroendocrinology 2000 71: 68–78.
Bernardis LL, Bellinger LL . The dorsomedial hypothalamic nucleus revisited: 1998 update Proc Soc Exp Biol Med 1998 218: 284–306.
Kuhn CM, Cochrane C, Feinglos MN, Surwit RS . Exaggerated peripheral responses to catecholamines contributes to stress-induced hyperglycemia in the ob/ob mouse Pharmacol Biochem Behav 1987 26: 491–495.
Bailey CJ, Flatt PR . Adrenoceptor-mediated control of glucose homeostasis in obese hyperglycemic (ob/ob) mice Diabetes Res 1990 14: 87–89.
Leigh FSM, Kaufman LN, Young JB . Diminished epinephrine excretion in genetically obese (ob/ob) mice and monosodium glutamate-treated rats Int J Obes Relat Metab Disord 1992 16: 597–604.
Carey RM, Van Loun GR, Baines AD, Kaiser DL . Suppression of basal and stimulated noradrenergic activities by the dopamine agonist bromocriptine in man J Clin Endocrinol Metab 1983 56: 595–602.
Vila E, Badia E, Jane F . Effects of bromocriptine on catecholamine receptors mediating cardiovascular responses in the pithed rat J Auton Pharmac 1985 5: 125–130.
Mannelli M, Delatali G, De Feo ML, Maggi M, Cuomo S, Piazzini M, Guazzelli R, Serio M . Effects of different dopaminergic antagonists on bromocriptine-induced inhibition of norepinephrine release J Clin Endocrinol Metab 1984 59: 74–78.
Jackson DM, Ross SB, Hashizume M . Further studies on the interaction between bromocriptine and SKF38393 in reserpine and alpha methyl-para-tyrosine-treated mice Psychopharmacology 1988 94: 321–327.
Scislowski PWD, Tozzo E, Zhang Y, Phaneuf S, Prevelige R, Cincotta AH . Biochemical mechanisms responsible for the attenuation of diabetic and obese conditions in ob/ob mice treated with dopaminergic agonists Int J Obes Relat Metab Disord 1999 23: 425–431.
Zhang Y, Scislowski PWD, Prevelige R . Bromocriptine/SKF38393 treatment ameliorates dyslipidemia in ob/ob mice Metabolism 1999 48: 1033–1040.
Liang Y, Matschinsky FM . Content of CoA-esters in perifused rat islets stimulated by glucose and other fuels Diabetes 1991 40: 327–333.
Liang Y, Matschinsky FM. Mechanisms of action of nonglucose insulin secretagogues . A Rev Nutr 1994 14: 59–81.
Cooper JR, Bloom FE, Roth RH (eds) . The biochemical basis of neuropharmacology, 6th edn. Oxford University Press: Oxford 1991.
Mukhherjee B, Chatterjee AK, Bhatia GS et al. Effect of epinephrine and norepinephrine on immuno-reactive insulin secretion from isolated islets of Langerhans Biochem Pharmac 1985 34: 985–987.
Ismail NA, El-Denshary ES, Idahl LA, Lindstrom P, Sehlin J, Taljedal IB . Effects of alpha-adrenoceptor agonists and antagonists on insulin secretion, calcium uptake, and rubidium efflux in mouse pancreatic islets Acta Physiol Scand 1983 118: 167–174.
Niddam R, Angel I, Bidet S, Langer SZ . Pharmacological characterization of alpha-2-adrenergic receptor subtype involved in the release of insulin from isolated rat pancreatic islets J Pharmacol Exp Ther 1990 254: 883–887.
Meier AH, Cincotta AH . Circadian rhythms regulate the expression of the thrifty genotype/phenotype Diabetes Rev 1996 4: 464–487.
Bjorntorp P, Holm G, Rosmond R . Hypothalamic arousal, insulin resistance and type 2 diabetes mellitus Diabetic Med 1999 16: 373–383.
Lonnquist F, Thorne A, Nilsell K, Hoffstadt J, Arner P . A pathogenic role of visceral fat β3-adrenoceptors in obesity J Clin Invest 1994 95: 1109–1116.
Scherrer U, Owlya R, Lepori M . Body fat and sympathetic nerve activity Cardiovasc Drugs Ther 1996 10: 215–222.
Grassi G, Cattaneo BM, Seravalle G, Colombo M, Cavagnini F, Mancia G . Obesity and sympathetic nervous system Blood Pressure 1996 5 (Suppl 1): 43–46.
Acknowledgements
We gratefully acknowledge the excellent technical assistance of Sussie Castro, Lisa Garrett and Jennifer Joslin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, Y., Cincotta, A. Increased responsiveness to the hyperglycemic, hyperglucagonemic and hyperinsulinemic effects of circulating norepinephrine in ob/ob mice. Int J Obes 25, 698–704 (2001). https://doi.org/10.1038/sj.ijo.0801614
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0801614
Keywords
This article is cited by
-
Neuroendocrine and metabolic components of dopamine agonist amelioration of metabolic syndrome in SHR rats
Diabetology & Metabolic Syndrome (2014)