Abstract
Five patients with inv dup(15) chromosomes were investigated with molecular probes on proximal 15q to determine the parental origin and extent of the duplicated segment. Cytogenetic investigation showed that four patients carried one and a fifth patient had two extra chromosomes derived from number 15 in all cells. In situ hybridization with a chromosome 15 library and a centromere 15 probe confirmed that the entire inv dup chromosomes were derived from chromosome 15. Molecular analysis using probes mapping in the region deleted in Prader-Willi syndrome (PWS) and Angelman syndrome (AS) patients implied that in at least two patients the extra chromosomes were asymmetric with one copy of the PWS region on the extra marker chromosome but two copies of the region centromeric to the PWS region. Three other cases had an inv dup(15) with two extra copies of the PWS region, but in one of these, heteromorphisms clearly demonstrated that the two centromeres derived from two different chromosomes. The inv dup(15) presumably resulted from an illegitimate recombination event between two different chromosomes 15 in most or all of these cases. All patients showed a maternal origin of the duplicated chromosome. The clinical severity appears to be associated with dosage of the PWS/AS region rather than with differences in the extent of the duplicated segment.
Similar content being viewed by others
Introduction
The presence of a small extra chromosome derived from chromosome 15 is a relatively common finding and is associated with a variable clinical phenotype. Growth is normal in two-thirds of the patients, and frequently found features include moderate to severe mental retardation, seizures, poor motor coordination, behavioral problems, and mild dysmorphic features [1].
In a summary of inv dup(15) cases reported in the literature, Maraschio et al. [2] differentiate two types of cytogenetically distinguishable inv dup(15) cases. The first type includes metacentric and almost entirely heterochromatic additional inv dup(15)(pter → q11::q11 → pter) chromosomes. These are found in normal individuals and on rare occasions are reported in patients with the Prader-Willi syndrome (PWS). According to studies of cytogenetic markers, the inv dup chromosomes in PWS patients seem to be derived from the same chromosome, whereas those in normal individuals are preferentially derived from two different chromosomes 15. Patients with the second catagory of inv dup(15) chromosomes, which include duplications of 15(pter → q12::q12 → pter) or 15(pter → q11::q13 → pter), show varying degrees of mental retardation but generally no physical abnormalities. Cases in this group also have predominantly different markers on either end of the rearranged chromosome. Maraschio et al. [2] also pointed out that most inv dup(15) chromosomes originate from the mother and that there is an association with increased maternal age.
The presence of two extra marker chromosomes derived from 15 is rare. It has been previously reported as a mosaicism in a fetus which was aborted [3] and in a retarded child [4]. Two smaller inv dup(15)(pter → q11::q11 → pter) chromosomes have twice been seen in infertile males [5, 6]. One 48, XY, +15q−, +mar(15) patient showed severe mental and motor retardation, but the mar(15) chromosome was also present in the normal father and the patient’s phenotype was probably due only to the additional 15q− chromosome [7].
Proximal chromosome 15q is known to undergo parental imprinting, since lack of the paternally derived segment of 15q11−913 from the father, either by deletion or uniparental disomy, results in the PWS [8–10], and lack of the same maternally derived region results in the Angelman syndrome (AS) [11–13]. Thus, the phenotype of inv dup(15) patients might be determined by both the extent of the duplicated region and the parental origin.
The extra chromosomes of several inv dup(15) patients have previously been shown to include duplication of one or more probes from the PWS/AS deletion region [14–16]. We report here on more extensive molecular analyses and clinical reports of five inv dup(15) patients. The extent of the duplicated region and mode of formation of the inv dup(15) chromosomes are investigated, and the relationship between genetic and clinical findings is discussed.
Case Reports and Methods
Patients
Patient A, a male (from Zürich), was born at term with a birth weight of 3,920 g and length of 52.0 cm (fig. 1). The mother and father were 28 and 34 years old, respectively, at his birth. One older sister is healthy and normal. During pregnancy with the patient, the mother recorded normal fetal movements. Breast-feeding lasted for 6 months without difficulty. Smiling was not observed until age 6 months. Seizures set in at age 11 months, at which time the patient’s psychomotor age was estimated to be at about 2 months. An EEG showed multifocal epilepsy, and a brain computed-tomographic scan disclosed brain atrophy. Treatment with Rivotril was initiated and the epilepsy temporarily improved but attacks became more frequent starting at 2 years 10 months.
Examination at age 3½ years showed severe motor and mental retardation. The patient was severely hypotonic, had diminished spontaneous motor activity, did not speak or walk, and had poor head control. Weight and length were around the 50th percentile, and head circumference was between the 50th and 75th percentile. A relatively adipose face and trunk as compared to limbs was noted. Neurologically, he showed signs of mixed hypotonic and spastic cerebral palsy. The scrotum was small, and the left testis was undescended. Despite treatment with the antiepileptic medications Rivotril, Mysoline and Phenytoin, he has occasional seizures. There was hypertrophy of the gums. Presently, at age 9 years, the patient does not sit alone, does not speak, is completely incontinent and wheelchair bound. Physical growth is still within normal limits.
The clinical details (at 13 years of age) of patient B, a male (from Zürich), have been published previously [11]. He was born at term with normal birthweight (3,250 g) and length (48 cm). At his birth, the mother was 32 and the father 35 years old. He has three younger siblings who are normal. He sat at 18 months and walked at 2½ years of age. At age 13 years, he showed normal growth, poor motor coordination with ataxic gait, mental retardation, and very aggressive and hyperactive behavior. Facial features included inner epicanthic folds, deep-set eyes with downslanting palpebral fissures, and poorly formed ears. The patient was last examined at 18 years old and measured 1.82 m in height (taller than his parents), 75 kg in weight and 55 cm in head circumference. He lives in an institution for moderately to severely mentally retarded males with behavioral problems.
Patient C, a female (from Zürich), was ascertained through prenatal diagnosis (fig. 1). Placental biopsy was performed because of intrauterine growth retardation and polyhydramnios noticed at the 30th week of pregnancy. The mother had previously had an extrauterine pregnancy at age 25 and a spontaneous abortion at age 30. The daughter was born at 38 weeks when the mother was 33 and the father was 39 years of age. She was less than the 10th percentile at birth for weight (1,790 g), length (43.5 cm), and head circumference (30 cm).
The patient had mild cyanosis, poor head control, and mild hypotonia. Plump hands and feet, and small, low-set ears were also noted. No feeding difficulties were initially apparent; however, at age 6 weeks vomiting set in and became serious. Pylorotomy was performed at 8 weeks. At this time, probable seizures were first observed. At clinical examination at age 5 months, length (56.7 cm) and head circumference (38.0 cm) were below the 3rd percentile while weight (6,190 g) was at the 50th percentile. The following minor dysmorphisms were ascertained: a small anterior fontanelle, narrow forehead, full cheeks, almond-shaped eyes in a mongoloid position, epicanthic folds extending to the cheeks, small nose, a carp-shaped mouth with downturned corners. Fingers were in a flexion position, with hyperconvex nails, and halluces were retropositioned. Muscular tone of the trunk was diminished, but was increased in the limbs; head control was still poor. Her mental age corresponded approximately to that of a 6-week-old. An EEG at age 7 months showed an active multifocal epilepsy, and antiepileptic therapy was initiated.
Patient D, a male (from Las Palmas) weighed 3,400 g at birth (at term), at which time his parents were both 31 years of age. The first signs of an abnormality were noted at age 6 months when epileptic seizures began and an EEG showed hypsarrhythmia. Both a brother and a maternal aunt have also had epileptic seizures. At 3 years of age, his length (95 cm) was at the 3rd percentile. At age 5 years the following features were noted: microcephaly (head circumference 48.6 cm, below the 3rd percentile), inner epicanthic folds, ante-verted nares, irregularly shaped teeth, high palate, pectus excavatum, agenesis of the left kidney, and severe mental retardation. He cannot walk, falls over frequently and can only repeat isolated words. He is irritable and cries frequently.
Patient E, a male (from Reutte) is the first child of a father aged 30 and a mother of 32 years at his birth; a younger sister is healthy. The mother recorded diminished fetal activity. He was born at term with the following measurements: weight 3,020 g, length 49.0 cm, head circumference 33.0 cm. Perinatal course was normal, but subsequent development was delayed from the beginning: marked muscular hypotonia was noticed at 3 months of age, grasping at 7–8 months, free sitting and first steps at 2½ years. Onset of myoclonic, later also hypsarrhythmic, seizures occurred at 9 months. Repeated EEGs showed multifocal and hypsarrhythmic epilepsy. The epileptic activity was very difficult to control by antiepileptic treatment. Subsequently, he lost the ability for free sitting, standing and walking again. At clinical examination at 11½ years of age he presented as a profoundly mentally retarded, adipose, bedridden, amimic, hypotonic, completely incontinent and epileptic male showing stereotypic movements. Length was 1.24 m (below the 3rd percentile), weight was 49 kg (97th centile) and head circumlerence was 51.0 cm (just above the 3rd percentile). Apart from strabismus convergens, hyperopia with astigmatism, irregular position of teeth and gum hypertrophy he does not show minor dysmorphic signs. Computed tomography of the skull and cerebrum revealed normal findings as did brainstem audiometry.
Cytogenetics
Peripheral blood lymphocytes from patients and parents were cultured using standard techniques. Metaphase preparations were stained with GTG, RTA, CBG, Distamycin A DAPI and AgNO3 staining techniques.
A chromosome-15-specific library (pBS-15 plasmid library obtained from Lawrence Livermore National Laboratory), ptra25, a centromere-specific alpha-satellite probe for chromosome 15 [18], and ML34, a single-copy probe mapping to the proximal region of the PWS deletion region, were used for in situ hybridization. The probes were labelled with biotinylated-14-dATP and hybridized following the protocol of Pinkel et al. [19].
DNA Analysis
Molecular studies on patients and parents were undertaken using the polymorphic probes in the PWS region (15q11–q13): pIR39 (D15S18); pML34 (DI5S9); p3–21 (DI5S10); pIR4-3 (DI5S11); pIR10-1 (D15S12), and p189-1 (D15S13) [9, 14, 20] and 28β3-H3 [21]. In addition, the probes pCMW-1 (D15S24) [22] recognizing a VNTR located just distal to the PWS region in 15q13, pMS-620 [23] recognizing a VNTR in the telomeric region of chromosome 15, and pMS1-14 (D15S1) [24] located to 15q14–q22 were used. A microsatellite at the GABRB3 locus was analyzed using the polymerase chain reaction [25].
Isolation of genomic DNA from blood, hybridization of probes, and densitometric analysis were performed as reported elsewhere [10]. Because absorbance readings are not always linear with respect to dosage (i.e. a sample with four copies of a locus may show either less or more than twice the absorbance of an individual with two copies depending on band intensity), distinguishing between three and four copies when the patient is homozygous is difficult. Although most RFLP alleles should hybridize to probes with equal intensity, in some cases there can be a significant difference which must be taken into account. For example the 9.0-kb BgIII allele with p39 (DI5S18) hybridizes with about twice the intensity in all normal heterozygotes as the 8.2-kb allele. Gel-blotting conditions may also influence the signal intensities.
Estimating the dosage of 15q11–13 probes in the patients presented here relied on two approaches. The ratio of signal intensities for the two alleles could be compared at loci where the patient was heterozygous and standardized to the ratio in normal controls. Thus if the patient has three copies at a particular locus, there should be an approximately 2:1 ratio of alleles, and if the patient has four copies there should be either a 2:2 or an approximately 3:1 ratio of alleles. To differentiate a 2:1 from a 3:1 copy ratio, we assume linearity of band intensities versus optical density. Dosage of each band was also calculated independently by hybridizing a constant probe from another chromosome and comparing to heterozygous and homozygous parents and controls. Enzyme/probe combinations were usually repeated at least once to verify the results.
Results
Cytogenetics
The karyotypes of patients A (fig. 2), B, D and E contained one bisatellited extra chromosome without mosaicism. The karyotype of patient C (fig. 3) showed two apparently identical bisatellited extra chromosomes in all analyzed mitoses.
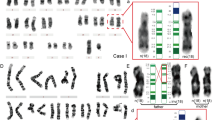
Cytogenetic results for patient A. a C banding: the inv dup(15) is indicated by an arrow. b Q banding: the inv dup(15) and both normal 15s are indicated by arrows. c AgNO3 staining: note the absence of NORs on one end of the inv dup(15) and on one normal 15 (indicated with arrows). d Dystamycin A DAPI staining: note the poorly DAPI-staining centromeres on one end of the inv dup(15) and on one normal 15.
Cytogenetic results for patient C. a C banding: both inv dup(15)s are indicated by arrows. b Q banding: both inv dup(15)s are indicated by arrows. c G-banded chromosomes: both the normal and inv dup(15)s are indicated by arrows. d Dystamycin A DAPI staining: note the presence of DAPI-positive centromeres on both ends of the inv dup(15)s.
CBG staining was done for patients A, B and C and disclosed a second inactive centromere in all extra chromosomes. Silver staining showed that the markers in patients B and C contained two NOR regions, one at each end, whereas one end of the marker and one normal chromosome 15 in patient A lacked an NOR region. Distamycin A DAPI staining showed a similar pattern: both marker ends and both normal 15 s were DAPI positive in patients B, C, D and E but one marker end and one normal chromosome 15 were DAPI negative (or only weakly staining) in patient A. His mother also carried a DAPI-negative chromosome 15 apparently identical to that in the child.
In situ hybridization of a centromere probe and a chromosome 15 library showed that the marker chromosomes were derived from chromosome 15 in all patients. The centromere probe, however, hybridized weakly or not at all to the DAPI-negative centromeres in patient A. As the DAPI staining in the mother of patient A showed a similar DAPI-negative chromosome 15, we assume that this is an individual variation and not related to the patient’s abnormal phenotype.
In situ hybridization with ML34 (a 6.2-kb insert in pBR322) gave clear results only for patients A and D, in whom two copies of this probe on the inv dup(15) chromosome could be visualized. Patient B probably has only one copy of this probe: of 50 metaphases analyzed, 28 showed no signal, 19 showed two fluorescent points (one on each chromatid), and 3 cells appeared to have three points on the inv dup(15) chromosome. However, a poor signal-to-noise ratio makes the results from this case difficult to interpret. Patients C and E were not hybridized with this probe.
Molecular A nalysis
The results for patient A (table 1) indicate that four copies of the entire PWS deletion region are present, but the duplicated region does not extend as far as pCMW-1 in this patient (fig. 4). The molecular results are in agreement with the cytogenetic finding that the extra chromosome is derived from two different maternal chromosomes.
Patient B has 3 copies of the loci recognized by probes 3–21 and 189-1. For probe p189-1, the 3.8-kb allele is equivalent in dosage to two copies in a normal homozygote whereas the 2.0-kb allele is calculated to be present in one copy. Probe CMW-1 in this case is clearly of greater intensity than expected if only two copies were present (fig. 4). Thus, the duplicated region appears to extend at least to 15q13. The allele copy ratio at D15S18 detected by p39 is most compatible with four copies. Cytogenetic markers indicate that the inv dup chromosome is derived from two different maternal chromosomes. Molecularly, assuming a maternal origin, both maternal alleles in the PWS region have been transmitted to the child, one on the normal 15 and one on the extra 15.
Patient C provides the most interesting data as there are two inv dup(15) chromosomes in each cell. The information from p189-1 shows that there are four copies of at least part of the PWS region (fig. 5). The two alleles at this locus are of equal intensity, and each allele corresponds to the dose present in a normal homozygote. The inv dup chromosomes can be identified as maternally derived as only the mother carries the 2.0-kb allele which is present in two copies in the patient. The duplicated region extends into 15q13 as four copies of the locus detected by pCMW-1 are present. The presence of six copies is estimated from the allele copy ratio at D15S18 (p39), the locus most proximal to the centromere. It can be inferred that the PWS region haplotype of the normal maternally derived chromosome 15 is not identical to that of the maternally derived extra chromosome. As both maternal haplotypes have been transmitted, a nondisjunction event must have occurred in the maternal meiosis with the formation of the inv dup(15) occurring either before or after this nondisjunction.
Molecular results for patient C. Hybridization of a TaqI filter indicates that four copies of p3–21 and two copies of each of the 3.8- and 2.1-kb alleles recognized by p189-1 are present, relative to the control probe pJ3.11 from chromosome 7. Three copies of a 2.0-kb allele and one copy of a 2.1-kb allele recognized by pCMW-1 appear to be present in patient C (densitometric reading was not done as the two alleles were too close in size). The mother was run on the same gel as the father and child. Her bands for CMW-1 are faint but are similar in size to the alleles in patient C.
Patients D and E are similar to patient A, having four copies of the PWS region, except that the duplicated region includes pCMW-1. A maternal origin of the extra chromosome was also found in patient D (based on GABRB3 results) and in patient E (based on results from p189-1, GABRB3 and pCMW-1).
Discussion
The results from the patients presented here indicate that dosage of the PWS region is a major factor determining clinical severity of the inv dup(15) phenotype (see fig. 6). Case B with three copies of the PWS region was much more mildly affected than were cases A, C, D and E who all had four copies of this region, even though case B was duplicated for a larger segment (extending to pCMW-1) than was patient A. From these results we predict that cases with one extra maternal copy of the PWS region will show moderate mental retardation and only mild physical handicaps (variation in phenotype may be due to extent of duplication or particular alleles inherited). Cases with two extra maternal copies are likely to show more severe mental retardation, epilepsy, and severe motor retardation.
Molecular dosage analysis of six patients with a cytogenetic genotype 47, XX or XY, +inv dup(15)(q13) have been reported previously [14–16]. Two of these patients probably had 3 copies and four were reported to have 4 copies of the region normally deleted in PWS and AS. A maternal origin of the extra chromosome was established in two cases. Few clinical features were reported for these patients and only mental retardation is common to all. Table 2 summarizes results for patients for whom both molecular and clinical features were available. Poor growth was only noted for patient C, which might be due to either a greater extent of the tetrasomic region past the locus detected by pCMW-1 or hexasomic dosage of proximal 15q genes.
As no paternally derived inv dup(15) chromosomes large enough to include the PWS region have yet been reported, it is possible that fetuses with increased dosage of paternally imprinted genes in this region are not viable. However, meiotic nondisjunction occurs predominantly in females, and a paternally inherited inv dup(15) is expected to be a relatively rare event.
Cases with very small inv dup(15) chromosomes, where the duplication does not extend to the PWS region, should always be normal. The presence of the PWS in a few such cases is probably due to maternal disomy of the two normal chromosomes 15 and not to the presence of an extra chromosome. Two-thirds of inv dup(15)s arising by a postzygotic loss in a trisomy 15 will have a normal chromosome 15 from each parent and one third would show uniparental disomy (see fig. 7). As the majority of trisomies are maternal in origin, the most common findings should be a maternally derived inv dup(15) with biparental normal 15s or a paternally derived inv dup(15) plus maternal disomy for the complete chromosome 15. This hypothesis is compatible with cytogenetic studies indicating that the inv dup(15)s found in normal and PWS subjects are similar in size and are too small to include the PWS gene region; the majority of normal subjects have a maternally derived extra chromosome whereas in the one informative PWS patient, the inv dup(15) was of paternal origin [2]. As the patients in the present study have two to three maternal copies of the PWS region and only one paternal copy, a simple dosage effect of maternal to paternal PWS genes cannot result in the PWS. This hypothesis would be very easy to test using the telomeric VNTR probe ms-620 to determine parental origin of the normal chromosomes in those PWS patients with additional marker chromosomes.
Possible outcomes of inv dup(15) resulting from a trisomy. When the inv dup(15) chromosome is large enough to include the PWS region (indicated by a black rectangle) then the individual is expected to be mentally retarded. A smaller marker chromosome should not result in an abnormal phenotype unless the loss of one of the three chromosomes from the trisomic cells results in uniparental disomy. As most trisomies are maternal in origin, maternal disomy (resulting in the PWS phenotype) should be more likely to occur than a paternal disomy (resulting in an AS phenotype).
If this latter hypothesis is correct and given that inv dup(15)(pter → q11::q11 → pter) is apparently associated much more than twice as often with a normal than a PWS phenotype, then the majority of such chromosomes in normal cases probably originate premeiotically (resulting in a biparental set of normal chromosome 15s) rather than postmeiotically. This would also explain the observation that inv dup(15)s associated with PWS usually have like markers (from a mitotic inversion/duplication event) whereas those with a normal phenotype or with a larger inv dup(15) usually have differing heteromorphisms (probably resulting from premeiotic or meiotic unequal crossing-over between homologous chromosomes).
Chromosome 15q11–13 is suspected to carry a high frequency of inverted repeat sequences [20]. An unequal crossover between inverted repeats within proximal 15q may explain both deletions and duplications of this region: deletions may occur when the recombination event is intrachromosomal and inv dup(15) chromosomes may result when the same recombination event is interchromosomal (either between homologues or sister chromatids).
Due to the small number of inv dup(15) chromosomes investigated molecularly, it is difficult to draw any major conclusions concerning their origin or phenotype associations. Reliably determining dosage by densitometry is labor intensive, which prohibits this method from yielding detailed results on a large number of patients. Nevertheless, a combination of banding techniques, in situ hybridization and RFLP analysis of additional patients would be useful to confirm the findings presented here.
References
Schinzel A: Catalogue of Unbalanced Chromosome Aberrations in Man. Berlin, Walther de Gruyter, 1984.
Maraschio P, Cuoco C, Gimelli G; Zuffardi O, Tiepolo L: Origin and clinical significance of inv dup(15); in Art D(ed): The Cytogenetics of Mammalian Autosomal Rearrangements. New York, Liss, 1988, pp 615–634.
Wisniewski LP, Doherty RA: Supernumary microchromosomes identified as inverted duplications of chromosome 15: A report of three cases. Hum Genet 1985;69:161–163
Van Dyke DL, Weiss L, Logan M, Pai GS: The origin and behavior of two isodicentric bisatellited chromosomes. Am J Hum Genet 1977;29:294–300
Martin-Lucas MA, Pérez-Castillo A, Abrisqueta JA: Infertility associated with two accessory bisatellited chromosomes. Hum Genet 1986;73:133–136
Manenti E: Two extra inv dup(15) chromosomes and male infertility: Second case. Am J Med Genet 1992;42:402–403
Voss R, Lerer I, Maftzir M, Cohen MM: Partial trisomy 15 in a male with severe psychomotor retardation (48, XY,+15q−, +mar(15)). Am J Med Genet 1982;12:131–139
Butler MG, Meaney FJ, Palmer CG: Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet 1986;23:793–809
Nicholls RD, Knoll JH, Glatt K, Hersh JH, Brewster TD, Graham JM, Wurster-Hill D, Wharton R, Latt SA: Restriction fragment length polymorphisms within proximal 15q and their use in molecular cytogenetics and the Prader-Willi syndrome. Am J Med Genet 1989;33:66–77
Robinson WP, Bottani A, Yagang X, Balakrishnan J, Binkert F, Mächler M, Prader A, Schinzel A: Molecular, cytogenetic, and clinical investigations of Prader-Willi syndrome patients. Am J Hum Genet 1991;49:1219–1234
Knoll JHM, Nicholls RD, Magenis RE, Graham JM Jr, Lalande M, Latt SA: Angelman and Prader-Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am J Med Genet 1989;32:285–290
Magenis RE, Toth-Fejel S, Allen LJ, Black M, Brown MG, Budden S, Cohen R, Friedman JM, Kalousek D, Zonana J, Lacy D, La Franchi S, Lahr M, Macfarlane J, Williams CPS: Comparisons of the 15q deletions in Prader-Willi and Angelmans syndromes: Specific regions, extent of deletions, parental origin, and clinical consequences. Am J Med Genet 1990;35:333–349
Williams CA, Zori RT, Stone JW, Gray BA, Cantu ES, Ostrer H: Maternal origin of 15q11–13 deletions in Angelman syndrome suggests a role for genomic imprinting. Am J Med Genet 1990;35:350–353
Tantravahi U, Nicholls RD, Stroh H, Ringer S, Neve RL, Kaplan L, et al: Quantitative calibration and use of DNA probes for investigating chromosome abnormalities in the Prader-Willi syndrome. Am J Med Genet 1989;33:78–89
Nicholls RD, Knoll JHM, Butler MG, Karam S, Lalande M: Genetic imprinting suggested by maternal heterodisomy in non-deletion Prader-Willi syndrome. Nature 1989;342:281–285
Shibuya Y, Tonoki H, Kajii N, Niikawa N: Identification of a marker chromosome as inv dup(15) by molecular analysis. Clin Genet 1991;40:233–236
Schinzel A: Particular behavioral symptomology in patients with rarer autosomal chromosome aberrations; in Schmid W, Nielsen J (eds): Human Behavior and Genetics. Amsterdam, Elsevier, 1981, pp 195–210.
Choo KH, Earle E, Vissel B, Filby RG: Identification of two distinct subfamilies of alpha satellite DNA that are highly specific for human chromosome 15. Genomics 1990;7:143–151
Pinkel D, Landegent J, Collins C, Fuscoe J, Segraves R, Lucas J, Gray JW: Fluorescence in situ hybridization with human chromosome specific libraries. Detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci USA 1988;85:9138–9142
Donlon TA, Lalande M, Wyman A, Bruns, Latt SA: Isolation of molecular probes associated with the chromosome 15 instability in the Prader-Willi syndrome. Proc Natl Acad Sci USA 1986:83:4408–4412.
Wagstaff J, Knoll JHM, Fleming J, Kirkness EF, Martin-Gallardo A, Greenberg F, Graham JM, Menninger J, Ward D, Venter JC, Lalande M: Localization of the gene encoding the GABAA receptor β3 subunit to the Angelman/Prader-Willi region of human chromosome 15. Am J Hum Genet 1991;49:330–337
Rich DC: Highly polymorphic locus D15S24 (CMW-1) maps to 15pter-q13. Nucleic Acids Res 1988;16:8740.
Armour JAL, Povey S, Jeremiah S, Jefferys AL: Systematic cloning of human minisatellites from ordered array chromatid libraries. Genomics 1990;8:501–512
Barker D, Schafer M, White R: Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell 1984;36:131–138
Mutirangura A, Ledbetter SA, Kuwano A, Chinault AC, Ledbetter DH: Dinucleotide repeat polymorphism at the GABAA receptor β3 (GABRB3) locus in the Angelman/Prader-Willi region (AS/PWS) of chromosome 15. Hum Mol Genet 1992;1:67.
Acknowledgements
We would like to thank Dr. M. Lalande for the 15q11.1–q12 probes. We would also like to thank I. Wälti, K. Schryber, F. Bernasconi and P. Tschepper for excellent technical assistance and the parents and patients for their cooperation.
This project was supported by the Roche Research Foundation (AS), Swiss National Foundation grant B 2006.295.3412 (WPR and AS), and the Julius Klaus Foundation (AS).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robinson, W.P., Binkert, F., Giné, R. et al. Clinical and Molecular Analysis of Five Inv Dup(15) Patients. Eur J Hum Genet 1, 37–50 (1993). https://doi.org/10.1159/000472386
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1159/000472386
Key Words
This article is cited by
-
The inv dup (15) or idic (15) syndrome (Tetrasomy 15q)
Orphanet Journal of Rare Diseases (2008)
-
Recurrent rearrangements in the proximal 15q11–q14 region: a new breakpoint cluster specific to unbalanced translocations
European Journal of Human Genetics (2007)
-
Characterization of an Autism-Associated Segmental Maternal Heterodisomy of the Chromosome 15q11–13 Region
Journal of Autism and Developmental Disorders (2007)
-
Cytogenetic abnormalities and fragile-x syndrome in Autism Spectrum Disorder
BMC Medical Genetics (2005)
-
Supernumerary marker chromosomes in man: parental origin, mosaicism and maternal age revisited
European Journal of Human Genetics (2005)