Abstract
Granular Groenouw type I (CDGG1) and lattice type I (CDL1) corneal dystrophies are two distinct potentially blinding conditions. These two entities were recently mapped to a region on chromosome 5q. We have investigated 2 families of Swiss origin with CDGG1 and CDL1 by linkage analysis. Our data show a maximum lod score of 5.38 at θ = 0.00 for marker D5S393 in CDL1 and 4.17 at θ = 0.00 for D5S658 in CDGG1. When combined, these families show a maximum lod score of 9.22 for D5S393 at θ = 0.00. This confirms previous reports. Furthermore, we describe a recombination centromeric to D5S399 in a member of the CDL1 family. Haplotype analysis in the 4 branches of the CDGG1 family demonstrated a common chromosomal region including D5S393 and D5S399 in all the affected members. By combining our data with previously reported mapping information and assuming that CDGG1 and CDL1 are allelic manifestations of the same gene, we can refine the location of the CDGG1/CDL1 gene to a 1-cM region on chromosome 5q. Using candidate genes in the 5q22-q32 interval, we investigated the possibility that mutations in the SPARC or LOX genes cause these corneal diseases. Several recombinations occurred between these two genes and CDGG1/CDL1 in our 2 families, thus excluding this hypothesis.
Similar content being viewed by others
Introduction
Granular Groenouw type I (CDGG1) and lattice type I (CDL1) corneal dystrophies are two distinct potentially blinding conditions characterized by progressive opacification of the cornea. These phenotypes are transmitted as nonpleiotropic, autosomal dominant traits with complete penetrance by the first decade of life.
In granular dystrophy, discrete white stromal opacities increase in number, extension and depth, and are referred as hyaline deposits with phospholipid content [1] and secondary accumulation of unesterified cholesterol [2]. Typically, these deposits give rise to multiple painful recurrent corneal erosions, and ultimately result in serious visual handicap around middle age. The superficial confluent form of granular dystrophy (superficial juvenile granular dystrophy) is probably due to a homozygous state [3].
In lattice dystrophy, grayish, linear, branching deposits progressively opacify the visual axis secondary to subepithelial and stromal accumulation of amyloid material. Symptoms are those of granular dystrophy but tend to appear at an earlier age. Lattice corneal dystrophy has been divided into three forms, namely the classic type I corneal amyloidosis strictly confined to the central cornea; the type II associated with familial systemic amyloidosis [4] caused by mutations in the gelsolin gene [5] in which lattice lines are more peripheral, and the type III [6] with an adult onset and a presumably autosomal recessive mode of inheritance (except for type IIIA which is autosomal dominant) [7]. Another rare recessive primary form of corneal amyloidosis characterized by central multinodular subepithelial opacities has been reported mainly in Japan under the name of gelatinous drop-like corneal dystrophy or familial subepithelial amyloidosis of the cornea [8, 9]. Finally, a combined granular-lattice corneal dystrophy has also been described under the name Avellino. This corneal dystrophy consists of both granular and lattice changes in the same eye [10].
Recently, Stone et al. [11] mapped three autosomal dominant corneal dystrophies (lattice type I, Groenouw type I and Avellino) to a region flanked by markers IL-9 and D5S436 on chromosome 5q. Although the cumulative lod score is impressive, only families with ancestors tracing back to Italy and Germany were studied. The analysis of additional families with different origins is therefore needed in order to assess whether these autosomal dominant corneal dystrophies are genetically homogeneous, i.e. are caused by mutations in the same gene. Following the report of Stone et al. [11], Eiberg et al. [12] reported linkage of Groenouw type I and Gregory et al. [13] confirmed the genetic localization of lattice corneal dystrophy type I to the same region of chromosome 5 in Danish and English families. We report here linkage analyses in 2 large families of Swiss origin and refine the genetic location of both diseases CDGG1/CDL1 to a 1-cM region between D5S393 and D5S396.
According to GDB (Genome Data Base, Johns Hopkins University School of Medicine, Baltimore, MD, accessible through WWW at the address: http://gdbwww.gdb.org/), several genes map to 5q22-q32. Based on their location and function, two of them, SPARC and lysyl oxidase (LOX), were considered as potential candidates. SPARC, an acidic calcium-binding glycoprotein involved in extracellular matrix assembly and LOX, an extracellular copper enzyme that initiates the crosslinking of collagen and elastin, were excluded due to several recombinations between them and the disease locus [14–17].
Materials and Methods
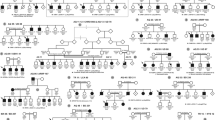
Complete eye examination was performed on all accessible living members of two large families of Swiss origin. Family LI is affected with lattice corneal dystrophy type I. The oldest affected member could be traced back to the end of the 19th century [18]. The second family (G 1 a-d) has granular corneal dystrophy Groenouw type I, and genealogical studies have revealed over 1,400 family members (fig. 1). The pedigree has been reported elsewhere [18, 19]. Blood samples were obtained after informed consent from 60 individuals (26 affected, 21 nonaffected and 13 spouses) and DNA was extracted from peripheral leukocytes. Molecular investigation did not start until complete clinical examination was performed and blood drawn from all family members. Haplotype analysis of affected and unaffected individuals was performed from PCR-amplified lymphocyte DNA using primers that flank microsatellites from the reported linked region (table 1). AU microsatellites have been reported by Généthon [20] and are accessible through WWW at http://www.genethon.fr/. PCR assays were carried out in a total volume of 10 µl using a Perkin-Elmer-Cetus 9600 machine. The reaction mixes and the PCR conditions were those described for the specific primers. One member of each pair of primers was FITC-labeled and PCR products were separated on an ALF automatic sequencer using Fragment Manager (Pharmacia Biotechnology, Sweden). Pedigrees and data were entered in Cyrillic (Cherwell Scientific Publishing Ltd., Oxford, UK) and directly exported in a format compatible with the computer program LINKAGE (version 5.2) [21]. Twopoint linkage analysis was done with all the markers assuming a dominant disease allele with a penetrance of 100% and a disease allele frequency of 0.0001. Allele frequencies of the markers were assumed to be equal [11] and the genetic distances used were obtained from Généthon [20].
SPARC was tested using a reported microsatellite. Its sequence and the PCR conditions have been described elsewhere [14–16]. For LOX, we used a reported PstI polymorphism in the first exon [17]. A region around this restriction site was amplified by PCR, digested with PstI, electrophoresed on Polyacrylamide gels and scored for the presence or absence of the PstI site.
Results
Although we have evidence that all 4 branches with Groenouw type I corneal dystrophy originate from a single ancestor, we analyzed each branch individually and then added the lod scores. Pairwise lod scores for CDGGl/CDLl locus and 8 microsatellite markers are given in table 1. Linkage analysis in the CDGG1 families showed a maximum lod score of 4.17 with marker D5S658 at a recombination fraction (θ) of 0.0 and 3.83 with D5S393 at θ = 0.0. In two branches of this family, markers D5S458 and D5S436 recombined with the disease, thus delineating an interval of 10 cM. When the affected haplotype from each branch was tabulated together, a genomic region extending from D5S393 to D5S396 was found to be common to all affected individuals (fig. 1). Based on data from Généthon, the sex-average genetic distance between these two markers is 1 cM [20].
Analysis of the family with lattice type I corneal dystrophy generated a maximum lod score for D5S393 of 5.38 at θ = 0.0. Interestingly, a recombination was found in one individual between D5S393 and D5S399. This healthy individual (2,782, fig. 2) inherited the affected haplotype from D5S399 to D5S436 from his mother (2,781), thus excluding the linkage of this region to the disease. The proximal part of the haplotype was homozygous. Therefore, a new marker (D5S666) was used to analyze this portion of the chromosome (fig. 2). If both diseases are allelic manifestations of a common gene, we could pool the data from both families. A maximum lod score of 9.21 at θ = 0.00 was then obtained for D5S393 and 6.45 at θ = 0.00 for D5S500.
In a second approach to locate the CDGGl/CDLl gene, we investigated the possible role of the SPARC and LOX genes. A microsatellite from the SPARC gene showed several recombinations with both Groenouw type I and the lattice type I dystrophies, as did a polymorphic PstI site in the LOX gene (data not shown).
Discussion
Corneal dystrophy Groenouw type I (CDGG1) and lattice type I (CDL1) have recently been linked to a 10-cM region on the long arm of chromosome 5 [11,12]. Gregory et al. [13] further mapped CDL1 to a region distal to D5S393. We report here additional linkage data of these two corneal dystrophies, refine the location of CDGG1 and CDL1 to a 1-cM within the region defined by D5S393 and D5S396, and exclude two candidate genes.
Results of the linkage analysis in the large family with CDL1 confirmed the previous mapping data by Stone et al. [11] and showed a maximum lod score with D5S393 and extended toward D5S658. One critical individual presented a recombination event between D5S393 and D5S399. This unaffected individual has inherited the same distal haplotype as his affected mother and was homozygous for the proximal part. Using an additional polymorphic marker (D5S666) located at the same position as D5S458, we were able to separate the 2 chromosomes and confirm the crossover. This would suggest that the CDL1 gene is proximal to D5S399. Of course, it is not possible at the present time to exclude a double recombination event. However, such a phenomenon would be very unlikely as the distance between D5S399, D5S500 and D5S396 is 0.00 cM. Another explanation could be that this nonaffected patient is, in fact, a nonpenetrant carrier. Here again, this is unlikely as it would be the first reported case of nonpenetrance in this disease. If one takes into account the proximal boundary set by Gregory et al. [13] at D5S393, the critical region for CDL1 is now 1 cM which, translated into physical distance, would be about 1 Mb, well within the clonable range.
Linkage analysis of the CDGG1 families showed the highest lod score at marker D5S658 and D5S393 with recombination at D5S458 and D5S436 thus defining a 10-cM region when each family was analyzed individually. However, if these families are analysed together, we can establish a common haplotype for the markers D5S393-D5S399-D5S500-D5S396. Interestingly, this common region is the same as the one defined by the CDL1 family. Three corneal dystrophies map to the same region on chromosome 5q [11] and it is not yet known whether they represent different allelic mutations of the same gene or a mutation in three adjacent genes. The linkage analysis reported here suggests that CDGG1 and CDL1 are identical or are within a common 1-cM region.
References
Rodrigues MM, Streeten BW, Krachraer JH, Laibson PR, Salem N, Passoneau J, Chock S: Microfibrillar protein and phospholipid in granular corneal dystrophy. Arch Ophthalmol 1983;101:802–810
Rodrigues MM, Kruth HS, Rajagopalan S, Jones K: Unesterified cholesterol in granular, lattice, and macular dystrophies. Am J Ophthalmol 1993;115:112–114
Ridgway AEA, Moller HU: Genetics of granular dystrophy. Ophthalmology 1992;99:1753.
Meretoja J: Familial systemic paramyloidosis with lattice dystrophy of the cornea, progressive cranial neuropathy, skin changes, and various internal symptoms. Ann Clin Res 1969; 1: 314–324.
de la Chapelle A, Tolvanen R, Boysen G, Santavy J, Bleeker-Wagemakers L, Maury CP, Kere J: Gelsolin-derived familial amyloidosis caused by asparagine or tyrosine substitution for aspartic acid at residue 187. Nat Genet 1992;2:157–160
Hida T, Tsubota K, Kigasawa K, Murata H, Ogata T, Akiya S: Clinical features of a newly recognized type of lattice corneal dystrophy. Am J Ophthalmol 1987;104:241–248
Stock EL, Feder RS, O’Grady RB, Sugar J, Roth SI: Lattice corneal dystrophy type IIIa; clinical and histopathologic correlations. Arch Ophthalmol 1991;109:354–358
Nagataki S, Tanishima T, Sakomoto T: A case of primary gelatinous drop-like corneal dystrophy. Jpn J Ophthalmol 1972;16:107–116
Shimazaki J, Hida T, Inoue M, Saito H, Tsubota K: Long-term follow-up of patients with familial subepithelial amyloidosis of the cornea. Ophthalmology 1995;102:139–144
Holland EJ, Daya SM, Stone EM, Folberg R, Dobler AA, Cameron JD, Doughman DJ: Avellino corneal dystrophy. Clinical manifestations and natural history. Ophthalmology 1992;99:1564–1568
Stone EM, Mathers WD, Rosenwasser GOD, Holland EJ, Folberg R, Krachmer JH, Nichols BE, Gorevic PD, Taylor CM, Streb LM, Fishbaugh JA, Daley TE, Sucheski BM, Sheffield VC: Three autosomal dominant corneal dystrophies map to chromosome 5q. Nat Genet 1994;6:47–51
Eiberg H, Moller HU, Berendt I, Mohr J: Assignment of granular corneal dystrophy Groenouw type I (CDGG1) to chromosome 5q. Eur J Hum Genet 1994;2:132–138
Gregory CY, Evans K, Bhattacharya SS: Genetic refinement of the chromosome 5q lattice corneal dystrophy type I locus to within a 2 cM interval. J Med Genet 1995;32:224–226
Swaroop A, Hogan BLM, Francke U: Molecular analysis of the cDNA for human SPARC/ Osteonectin/B-40: sequence, expression, and localisation of the gene to chromosome 5q31-q33. Genomics 1988;2:37–47
Goldblum SE, Ding W, Funk SE, Sage EH: SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function. Proc Natl Acad Sei USA 1994;91: 3448–3452.
Dixon MJ, Dixon J, Houseal T, Bhatt M, Ward DC, Klinger K, Landes GM: Narrowing the position of the Treacher Collins syndrome locus to a small interval between three new microsatellite markers at 5q32-33.1. Am J Hum Genet 1993;52:907–914
Csiszar K, Mariani TJ, Gosin JS, Deak SB, Boyd CD: A restriction fragment length polymorphism results in a nonconservative amino acid substitution encoded within the first exon of the human lysyl oxidase gene. Genomics 1993;16:401–406
Hermann C: La dystrophie grillagée de la cornée: contribution clinique et anatomopathologique. Ophthalmologica 1943;112:350–363
Cuendet JF, Beuret-Niedzielsky A, Zografos L: Hérédité de la dystrophie granuleuse de la cornée (Groenouw I). Ophthalmologie 1989;3:265–266
Gyapay G, Morissette J, Vignal A, Dib C, Fizames C, Millasseau P, Marc S, Bernardi G, Lathrop M, Weissenbach J: The 1993–94 Généthon human genetic linkage map. Nat Genet 1994;7:246–339
Lathrop GM, Lalouel JM, Julier C, Ott J: Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sei USA 1984;81:3443–3446
Acknowledgements
We are indebted to our colleagues who helped us in obtaining blood samples from their patients: Drs. Ph. Ecoffey, P. Scherrer and M. Wahli in Château-d’Oex, M.-N. Vogt and B. Cochet in Aigle, D. Bourquin in Les Mosses, N. Mühlemann in Bex, A. Vilaseca in Carouge, M. Mottaz in Versoix, F. Membrez in Delémont. We also thank Ms. G. Martin, K. Kahlen and A. Pinto for technical assistance. This work was supported in part by a grant from the Ligue Suisse pour la Prévention et la Lutte contre la Cécité and grant No. 3200-043619.95 from the Swiss National Science Foundation. This paper is dedicated to the participating families.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Korvatska, E., Munier, F.L., Zografos, L. et al. Delineation of a 1-cM Region on Distal 5q Containing the Locus for Corneal Dystrophies Groenouw Type I and Lattice Type I and Exclusion of the Candidate Genes SPARC and LOX. Eur J Hum Genet 4, 214–218 (1996). https://doi.org/10.1159/000472201
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1159/000472201
Key Words
This article is cited by
-
Kerato-epithelin mutations in four 5q31-linked corneal dystrophies
Nature Genetics (1997)