Abstract
Aim:
To investigate the effects cannabidiol (CBD) on delayed-type hypersensitivity (DTH) reactions and antigen-induced T-cell cytokine expression.
Methods:
DTH was induced by subcutaneous ovalbumin (OVA) challenge to the footpads of mice sensitized with OVA. Inflammatory reactions were measured by footpad swelling and histological analysis. Antigen-induced cytokine expression by OVA-primed splenocytes was measured using ELISA and RT-PCR.
Results:
CBD (1-10 mg/kg) administration, in a dose-dependent fashion, significantly attenuated inflammatory reactions associated with DTH in the footpads of mice sensitized and challenged with OVA. Histological examination revealed that CBD suppressed the infiltration of T cells and macrophages, and the expression of interferon (IFN)-γ and tumor necrosis factor-α, two pro-inflammatory cytokines implicated in DTH in the inflammatory site. In contrast, the expression of interleukin (IL)-10 in the footpads was enhanced by CBD administration. In addition, CBD at concentrations devoid of cytotoxic effects (1-4 μmol/L) attenuated OVA-induced IFN-γ production by OVA-primed splenocytes, whereas IL-4 was unaffected.
Conclusion:
CBD curbs DTH reactions via suppressing the infiltration and functional activity of T cells and macrophages in the inflammatory site, suggesting a therapeutic potential for CBD for the treatment of type IV hypersensitivity.
Similar content being viewed by others
Introduction
Cannabis sativa, also known as marijuana, has a long history of medicinal and recreational uses. Cannabinoids are the active constituents contained in marijuana, among which Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are the most abundant psychotropic and non-psychotropic compounds, respectively1, 2, 3. Both Δ9-THC and CBD demonstrate a broad spectrum of pharmacological activities, such as anti-inflammation and immunomodulation4, 5, 6, 7, 8,9,10. The affinity of Δ9-THC to cannabinoid receptors contributes to its psychotropic property that limits its clinical application. In contrast, the non-psychotropic property of CBD renders it a promising candidate as a potential immunomodulatory agent.
Both Δ9-THC and CBD are found to affect the reactivity of T cells and macrophages11, 12, 13, 14, 15. The effect of CBD on T cells has been investigated extensively in vitro and in some murine models, such as type-1 diabetes and rheumatoid arthritis[7, 8, 10, 12, 16]. CBD attenuated the disease progression in non-obese diabetes mice, which was closely associated with a reduction of the plasma levels of the proinflammatory cytokines IFN-γ and TNF-α10, 16. Likewise, CBD diminished the proliferation and IFN-γ production by T cells in a model of collagen-induced arthritis12. In addition, we previously demonstrated that CBD markedly suppressed the serum production of antigen-specific antibodies in OVA-sensitized mice6. The ability of splenocytes of CBD-treated mice to produce IL-2, IL-4, and IFN-γ was also diminished6. Collectively, these results demonstrated that CBD modulated T cell-mediated immune responses, in which both T helper (Th)1 (ie, IFN-γ) and Th2 (ie, IL-4) cytokines were affected. However, it is currently unclear if CBD is effective in modulating hypersensitivity reactions.
Two subsets of Th cells, namely Th1 and Th2, play a pivotal role in regulating different arms of the adaptive immunity. Th1 cells primarily express IFN-γ, IL-2, and TNF-β that stimulate cytotoxic T cells and macrophages, thereby promoting cellular immune reactions. Th2 cells mainly produce IL-4, IL-10, and IL-13 that activate mast cells, eosinophils and B cells, which facilitate allergic and humoral immune responses17, 18, 19, 20. The imbalance of the development of Th1 and Th2 cells is involved in various immune disorders, such as hypersensitivity19, 21, 22, 23.
Given the therapeutic potential of CBD as an anti-inflammatory and immunomodulatory agent, and the reported effects of CBD on the expression of both Th1 and Th2 cytokines, the objective of the present study is to explore the therapeutic potential of CBD for DTH, a Th1 cell-mediated immunity in response to specific antigens. We report here that CBD markedly suppressed inflammatory reactions associated with DTH, which was closely associated with a down-regulation of the functionality of T cells and macrophages.
Materials and methods
Reagents and animals
All reagents were purchased from Sigma (St Louis, MO, USA) unless otherwise stated. Reagents used for immunohistochemical (IHC) staining were purchased from BioGenex Laboratories (San Ramon, CA, USA) and AbCam, Inc (Cambridge, MA, USA). Reagents and enzymes used for RT-PCR were purchased from Promega (Madison, WI, USA). ELISA sets for cytokine measurement were purchased from BD Biosciences (San Diego, CA, USA). Cannabidiol (CBD) was purchased from THC Pharm GmbH (Frankfurt, Germany). CBD was dissolved in absolute ethanol and stored at −20 °C. For cell culture studies, CBD was further diluted to the desired concentrations with RPMI-1640 medium (Hyclone, Logan, UT, USA). For animal studies, CBD was diluted with saline containing 10% Tween 20. Male BALB/c mice (5 weeks old) were obtained from the Animal Breeding Center of the National Taiwan University Hospital (Taipei, Taiwan) and housed in a temperature (25±2 °C), humidity (60%±20%) and light (12-h light/dark cycle)-controlled environment. Mice were supplied with standard food and water ad libitum.
Protocol of CBD administration, and OVA sensitization and challenge
The mice were randomly divided into the following 4 groups (4–5 mice/group; Figure 1): non-sensitized (NS), OVA-sensitized and challenged (OVA), vehicle-treated and OVA-sensitized and challenged (VH), and CBD-treated and OVA-sensitized and challenged (CBD). Except for the NS group, mice were sensitized with OVA by a subcutaneous injection with 0.1 mL of sensitization solution containing 10 μg OVA (0.5 mg/kg of body weight) and 200 μg alum (10 mg/kg; as adjuvant) in saline per mouse on day 0. CBD (1, 5 and 10 mg/kg) and/or vehicle (VH; 10% ethanol and 10% Tween 20 in saline) were daily administered into mice by intraperitoneal (ip) injection from days 6–10. The footpads of all mice were subcutaneously challenged with OVA (10 μg in 25 μL of saline) 1 h post the last drug administration. Delayed-type hypersensitivity (DTH) reactions represented by the degree of footpad swelling were measured by an electronic caliper before and 24 h after the OVA challenge. Following the measurement of swelling, footpads were isolated, fixed in 10% neutral buffered formalin for 2 days, and then immersed in a rapid decalcifier-solution (Merck, Darmstadt, Germany) at room temperature for 3 days. The tissue blocks were then embedded in paraffin and cut into 4–5 μm sections for IHC analysis. The animal experiments were approved by the Institutional Animal Care and Use Committee of the National Taiwan University.
Immunohistochemistry
Paraffin-embedded tissue sections were deparaffinized with xylene, and then rehydrated by immersing sequentially in 100%, 95 %, 90%, 80%, 60% ethanol for 5 min each for hydration. The hydrated slides were immersed in Trilogy (Cell Marque Corporation, Rocklin, CA, USA) at 121 °C for 15 min for antigen retrieval. The slides were then treated with 3% H2O2 for 15 min followed by blocking with normal horse serum for 1 h. Anti-mouse CD3, F4/80, IFN-γ, and TNF-α monoclonal antibodies were applied onto each section overnight at 4 °C, after which the slides were treated with super enhancer for 1 h, and then incubated with poly-HRP reagent for 1 h. For visualization, the slides were treated with the HRP substrate 3-amino-9-ethylcarbazole for 2 min followed by hematoxylin counter staining for 1 min. The number of positive signals (red color) was identified in contrast to the background counter stain (blue color) using the Image-Pro Plus version 5.1 software. Three measurements per footpad and 6−8 footpads per group were analyzed at 200-fold magnification.
Splenocyte cultures and cytokine measurement by ELISA
Each mouse was sensitized with OVA by intraperitoneal (ip) injection using 0.1 mL sensitization solution containing 20 μg OVA (1 mg/kg) and 2 mg alum (100 mg/kg) on day 0, and boosted on day 14. On day 15, the mice were euthanized and their spleens were aseptically isolated and made into single cell suspensions by pressing spleens with microtubes in culture dishes containing medium. The cell suspension was harvested and splenocytes were washed and resuspended in RPMI 1640 supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 5% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA). Splenocytes (5×106 cells/mL) were pretreated with CBD (0.25–4 μmol/L) and/or vehicle (VH; 0.1% ethanol) for 15 min, and then re-stimulated with OVA (100 μg/mL) for 72 h. CBD was present in the medium during the OVA stimulation. The supernatants were collected and assayed for IFN-γ and IL-4 production by ELISA as previously described6.
MTT assay
Splenocytes (5×106) were seeded into 96-well culture plates (0.1 mL/well), and received CBD pretreatment and OVA re-stimulation for 68 h as described above. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) stock solution (5 mg/mL in PBS) was added to each well (10 μL/well) and incubated for 4 h. At the end of incubation, the formed formazan was dissolved with 3 mol/L H2SO4, and then the plates were read at 570 nm, and at 630 nm as background.
Semi-quantitative RT-PCR
The total RNA of splenocytes was extracted using TRI reagent (Sigma) following the supplier's instructions. 100 ng total RNA of each sample was reverse transcribed into cDNA by 40 units MMLV reverse transcriptase using oligo-dT as the primer. The reverse transcription proceeded as the following: 42 °C for 15 min and 95 °C for 5 min. The PCR mixture containing PCR buffer, 4 mmol/L MgCl2, 6 pmol each of forward and reverse primers, and 2.5 units of Taq DNA polymerase was added to each cDNA sample. Samples were heated to 94 °C for 3 min and cycled 22–30 times at 94 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s followed by an additional step at 72 °C for 5 min. The PCR products were electrophoresed in 2% agarose gels and stained with ethidium bromide (2.5 μg/mL) for visualization. The DNA products were quantified by assessing the optical density of each band using the alpha imager 1200 digital imaging system (Alpha Innotech Crop, San Leandro, CA). The expression level of the housekeeping gene β-actin was employed as the control for semi-quantification of each target gene. Results were expressed as the ratio of the optical density of target genes/β-actin. The primers used are as follows: 5′-AACGAGGTCACAGGAGAAG-3′ and 5′-GTCTATCGATGAATCCAGGC-3′ for IL-4, 5′-CATGAAAATCCTGCAGAGCC-3′ and 5′-GGACAATCTCTTCCCCAGCC-3′ for IFN-γ, and 5′-AGGGAAATCGTGCGTGACATAAAA-3′ and 5′-ACTCATCGTACTCCTGCTTGCTGA-3′ for β-actin.
Statistic analysis
The mean±standard error (SEM) was determined for each treatment group in the individual experiments. Homogeneous data were evaluated by a parametric analysis of variance, and Dunnett's two-tailed t-test was used to compare treatment groups to the control group. P<0.05 was defined as statistically significant.
Results
CBD administration attenuated delayed-type hypersensitivity (DTH) reactions
To explore the potential of CBD as a therapeutic agent for Th1 cell-mediated immune disorders, we investigated the effect of CBD on DTH, in which antigen-specific Th1 cells are required for eliciting the hypersensitivity reaction. A murine model of DTH induced by OVA sensitization and challenges was employed. OVA challenge markedly increased the thickness of footpads in OVA-sensitized mice as compared to the NS group (Figure 2A; NS vs OVA), indicating a successful induction of DTH. The footpad swelling was significantly attenuated by CBD administration in a dose-dependent manner (Figure 2A; VH vs CBD). Consistent with the results of footpad swelling, histological examination using H&E staining revealed a heavy infiltration of mononuclear cells in subcutaneous tissues of the footpads in the OVA and VH groups, which appeared to be diminished in CBD (5 and 10 mg/kg)-treated groups (Figure 2B).
Attenuation by CBD administration of the footpad swelling and inflammatory cell infiltration induced by OVA challenge. BALB/c mice were treated as the protocol shown in Figure 1. DTH reactions in the footpad were induced by a subcutaneous OVA challenge. (A) The thickness of footpads was measured before and 24 h after the OVA challenge. The data are expressed as the mean±SEM of 6−8 samples per group. cP<0.01 compared to the NS group. eP<0.05, fP<0.01 compared to the VH group. (B) The tissue sections were stained with H&E (original magnification, ×100). Boxes show subcutaneous regions with heavy infiltration of mononuclear cells in the OVA and VH groups, which appears to be diminished in CBD (5 and 10 mg/kg)-treated groups. Results are representative of three independent experiments.
CBD administration attenuated the infiltration and cytokine expression by T cells and macrophages.
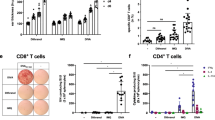
The effect of CBD administration on the type of infiltrated cells in the footpads was examined using IHC staining. As expected, a marked infiltration of CD3+ T cells (Figure 3A and Table 1) and F4/80+ macrophages (Table 1) was observed in the OVA group as compared to the NS group, confirming the involvement of T cells and macrophages in DTH. A comparable number of the CD3+ cells was observed between the OVA and VH groups, indicating that the VH per se did not affect the cell infiltration (Table 1). More importantly, CBD (1–10 mg/kg) administration significantly attenuated the number of the infiltrated CD3+ and F4/80+ cells in a dose-dependent manner (Table 1).
Immunohistochemical analysis of the infiltration of T cells and macrophages, and the expression of IFN-γ, TNF-α, and IL-10 in OVA-challenged footpads. BALB/c mice were treated as the protocol shown in Figure 1. Tissue sections of footpads were subjected to IHC staining for CD3, F4/80, IFN-γ, TNF-α, and IL-10. (A) Representative sections stained for CD3 are shown (original magnification, ×200). Cells with red signals around the blue nuclei indicate CD3+ cells. (B) Representative sections from the VH and CBD (10 mg/kg)-treated groups stained for F4/80, IFN-γ, TNF-α, and IL-10 are shown (original magnification, ×200). Quantitative data are shown in Table 1. Results are representative of three independent experiments.
We next examined whether CBD affected the expression of IFN-γ and TNF-α, two pro-inflammatory cytokines involved in the pathophysiology of DTH. Similar as the profile of T cell and macrophage infiltration, a strong positive signal was detected in subcutaneous tissues of the footpads in the OVA and VH groups, which was markedly attenuated by CBD administration (Figure 3B and Table 1). To further investigate the anti-inflammatory effect of CBD, the expression IL-10, a Th2 associated cytokine possessing anti-inflammatory activity, was examined. We revealed that CBD at the highest dose (10 mg/kg) significantly augmented the number of IL-10+ cells in the footpads (Figure 3B and Table 1).
A differential effect of CBD on the expression of IFN-γ and IL-4 induced by specific antigen
The above results showing that CBD administration attenuated the cytokine expression associated with DTH prompted us to further examine whether CBD directly influenced the expression of antigen-specific Th1/Th2 cytokines by T cells. OVA-primed splenocytes isolated from mice systemically sensitized with OVA were directly exposed to CBD (0.25–4 μmol/L) and/or vehicle (VH; 0.1% ethanol), and then re-stimulated with OVA (100 μg/mL) to induce cytokine expression. The level of Th1 and Th2 signature cytokines, IFN-γ and IL-4, respectively, in the supernatants was measured. Direct exposure of splenocytes to CBD (1–4 μmol/L) significantly attenuated OVA-induced IFN-γ production in a concentration-dependent manner (Figure 4A), whereas IL-4 was unaffected (Figure 4B). The range of CBD concentrations ≤4 μmol/L was used according to our previous results demonstrating the pro-apoptotic effect of CBD at concentrations ≥4 μmol/L in naïve splenocytes14. The results of our MTT assays confirmed that CBD ≤4 μmol/L produced no cytotoxicity, as evidenced by the lack of effect on the cell metabolic activity (Figure 4C). The level of cytokine in the supernatants of splenocytes without OVA re-stimulation was below the detection limit of ELISA (data not shown). The effect of CBD on the cytokine expression was further examined at the mRNA level. Consistent with the attenuation of protein production, the level of IFN-γ mRNA expression was markedly attenuated by CBD (2–4 μmol/L) treatment, whereas the level of IL-4 was unaltered in splenocytes re-stimulated with OVA (Figure 5).
The effect of CBD on antigen-induced production of IFN-γ and IL-4 and the metabolic activity in OVA-primed splenocytes. OVA-primed splenocytes (5×106 cells/mL) isolated from OVA-sensitized BALB/c mice were pretreated with CBD (0.25–4 μmol/L) and/or VH (0.1% ethanol) for 30 min followed by re-stimulation with OVA (100 μg/mL). After 72 h of culture, the level of (A) IFN-γ and (B) IL-4 in the supernatants was quantified by ELISA, and (C) the metabolic activity was determined using an MTT assay. The data are expressed as the mean±SEM of quadruplicate samples per group. bP<0.05, cP<0.01 compared to the VH group. Results are a representative of five independent experiments.
The effect of CBD on antigen-induced mRNA expression of IFN-γ and IL-4 by OVA-primed splenocytes. Splenocytes were treated with CBD and stimulated with OVA as described in Figure 4. After 24 h of culture, the total RNA was extracted and the mRNA expression of IFN-γ, IL-4 and β-actin was measured by RT-PCR. The data are expressed as the mean±SEM of three independent experiments. bP<0.05, cP<0.01 compared to the VH group.
Discussion
CBD is a promising anti-inflammatory and immunomodulatory phytocannabinoid currently under intensive investigation. Previous reports showed that CBD affected both humoral and cell-mediated immune responses in a number of experimental models6, 10, 12, 16, 24. In these reports, CBD demonstrated a broad spectrum of effects on the serum production of inflammatory cytokines and the expression of cytokines by lymphocytes activated by the T cell mitogen ConA. To date, evidence pertaining to the effect of CBD on hypersensitivity reactions and antigen-induced cytokine expression is limited. Hence, we investigated the in vivo effect of CBD on DTH, an immune response elicited by specific antigens. CBD demonstrated a markedly suppressive effect on OVA challenge-induced inflammatory reactions associated with DTH, including the footpad swelling and the infiltration of T cells and macrophages. In addition, an inhibitory effect of CBD on IFN-γ expression in the inflammatory site was revealed. Our in vitro experiments further showed that CBD possessed a directly suppressive effect on antigen-induced expression of IFN-γ, but not IL-4, by OVA-primed splenocytes. Collectively, these results clearly demonstrated a markedly suppressive effect by CBD on Th1 cell-mediated immune responses, suggesting a potential for CBD as an anti-inflammatory and immunomodulatory agent for managing type IV hypersensitivity.
The observed attenuation of T cell-mediated responses is in line with previous reports showing the immunosuppressive effect of CBD in two T cell-related murine models, including type-1 diabetes and collagen-induced arthritis10, 12, 16. In these models, a common feature of CBD-mediated effect is a marked attenuation of IFN-γ production and the subsequent immune/inflammatory responses elicited by this cytokine. In our studies, the pathophysiology of DTH involves both T cells and macrophages, in which IFN-γ produced by antigen-specific Th1 cells activates macrophages that subsequently trigger DTH reactions by secreting proinflammatory mediators, such as TNF-α25, 26. Our data showing the attenuated infiltration of macrophages and TNF-α expression in the footpads of CBD-treated mice indicated that CBD affected macrophages, the downstream inflammatory cells activated by T cells. These results are consistent with the nature of cell-mediated responses in the employed DTH model, and suggest that the effect of CBD may be mediated by down-regulating the activation and/or the recruitment of Th1 cells and macrophages in response to antigen challenges.
CBD enhanced the expression of IL-10 in OVA-challenged footpads, which is an anti-inflammatory cytokine associated with Th2 cells19, 20. Together with the attenuated IFN-γ expression, CBD induced a shift of the Th1/Th2 immunobalance toward the Th2 direction in the inflammatory site, suggesting a potential mechanism for CBD-mediated anti-inflammatory effect against DTH. However, the effect of CBD on IL-10 was not dose-dependent, in which CBD at 10 mg/kg was effective, whereas lower doses were not. Thus, the effect of CBD on DTH cannot be solely attributed to IL-10, other mechanisms should be considered. For example, CBD has been documented to modulate the turnover of endocannabinoids. CBD interfered with the metabolism of anandamide by inhibiting fatty acid amide hydrolase27. As anandamide has been reported to enhance IL-10 production by activated microglia28, and to protect neurons from inflammatory damage29, we speculate that the endocannabinoid system may play a role in the observed anti-inflammatory effect of CBD on DTH. More studies are required to decipher the potential role of endocannabinoids in CBD-mediated attenuation of DTH reactions.
We previously reported that CBD exhibited a broad spectrum of inhibitory effects on the expression of cytokines, including IFN-γ and IL-4 induced by ConA6. In the present study, we showed a contrasting effect of CBD between IFN-γ and IL-4 induced by the specific antigen OVA, in which only the Th1 cytokine IFN-γ was sensitive to CBD inhibition, suggesting that Th1 cells may be more sensitive to CBD. Many factors may account for the different results between our previous and current results. An obvious one is the mode of T-cell activation, in which a mitogen (ie, ConA) and a specific antigen (ie, OVA) were used in the previous and current studies, respectively. It is noticed that ConA-induced proliferation of splenocytes was inhibited by CBD administration6, 12; hence, the broad suppression of T cell cytokine expression by CBD reported in our previous report may be attributed to a general inhibition of cell proliferation. By comparison, the current study focused on the functional activities of antigen-stimulated T cells and the results showed that the metabolic activity of OVA-primed splenocytes re-stimulated with OVA was not influenced by CBD. Therefore, CBD-mediated differential modulation on antigen-induced expression of IFN-γ and IL-4 may not be attributed to a general cellular mechanism, such as inhibition of cell proliferation.
Another possible mechanism that may account for the direct suppression of IFN-γ by CBD is the induction of apoptosis, as we previously reported that CBD ≥4 μmol/L caused a direct pro-apoptotic effect in naive lymphocytes11, 14. However, the current study showed that CBD in a non-apoptotic concentration range (1−4 μmol/L) was effective to suppress IFN-γ, but did not affect OVA-induced IL-4 expression and the splenocyte metabolic activity. On the base of these results, the effect of CBD on antigen-induced IFN-γ expression is apparently not to be mediated by the induction of apoptosis. The precise mechanism for the differential sensitivity of the Th1 and Th2 cytokines to CBD is an intriguing issue to be addressed. It also requires further elucidation if CBD induces apoptosis in antigen-stimulated T cells.
In summary, the present study demonstrated that CBD exhibited a differential effect on the expression of antigen-induced Th1/Th2 cytokines and suppressed DTH reactions in OVA-sensitized and challenged mice. CBD attenuated the expression of IFN-γ, a key Th1 cytokine, whereas the Th2 cytokine IL-4 was unaffected. Results from the present study provide insights into the immunomodulatory effect of CBD, implicating that CBD is a potential therapeutic agent for the management of Th1-dominant immune disorders.
Author contribution
Dr Der-zen LIU designed the research; Chieh-min HU, Chung-hsiung HUANG, Shiaw-pyng WEY performed the research; Dr Tong-rong JAN coordinated the project and wrote the manuscript.
References
Mechoulam R . Marihuana chemistry. Science 1970; 168: 1159–66.
Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO . Cannabidiol--recent advances. Chem Biodivers 2007; 4: 1678–92.
Turner CE, Elsohly MA, Boeren EG . Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod 1980; 43: 169–234.
Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med 2009; 87: 1111–21.
Burstein SH, Zurier RB . Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J 2009; 11: 109–19.
Jan TR, Su ST, Wu HY, Liao MH . Suppressive effects of cannabidiol on antigen-specific antibody production and functional activity of splenocytes in ovalbumin-sensitized BALB/c mice. Int Immunopharmacol 2007; 7: 773–80.
Kaplan BL, Springs AE, Kaminski NE . The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem Pharmacol 2008; 76: 726–37.
Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, et al. The cannabinoid system and immune modulation. J Leukoc Biol 2003; 74: 486–96.
Klein TW . Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 2005; 5: 400–11.
Weiss L, Zeira M, Reich S, Har-Noy M, Mechoulam R, Slavin S, et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity 2006; 39: 143–51.
Lee CY, Wey SP, Liao MH, Hsu WL, Wu HY, Jan TR . A comparative study on cannabidiol-induced apoptosis in murine thymocytes and EL-4 thymoma cells. Int Immunopharmacol 2008; 8: 732–40.
Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA 2000; 97: 9561–6.
McCoy KL, Gainey D, Cabral GA . Delta-9-tetrahydrocannabinol modulates antigen processing by macrophages. J Pharmacol Exp Ther 1995; 273: 1216–23.
Wu HY, Chu RM, Wang CC, Lee CY, Lin SH, Jan TR . Cannabidiol-induced apoptosis in primary lymphocytes is associated with oxidative stress-dependent activation of caspase-8. Toxicol Appl Pharmacol 2008; 226: 260–70.
Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD . Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol 2002; 133: 124–31.
Weiss L, Zeira M, Reich S, Slavin S, Raz I, Mechoulam R, et al. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology 2008; 54: 244–9.
Abbas AK, Murphy KM, Sher A . Functional diversity of helper T lymphocytes. Nature 1996; 383: 787–93.
Flavell RA . The molecular basis of T cell differentiation. Immunol Res 1999; 19: 159–68.
Kidd P . Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev 2003; 8: 223–46.
Mosmann TR, Coffman RL . TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989; 7: 145–73.
Crow MK, Kirou KA . Interferon-alpha in systemic lupus erythematosus. Curr Opin Rheumatol 2004; 16: 541–7.
Gordon JN, Di Sabatino A, Macdonald TT . The pathophysiologic rationale for biological therapies in inflammatory bowel disease. Curr Opin Gastroenterol 2005; 21: 431–7.
Wu C, Yang G, Bermudez-Humaran LG, Pang Q, Zeng Y, Wang J, et al. Immunomodulatory effects of IL-12 secreted by Lactococcus lactis on Th1/Th2 balance in ovalbumin (OVA)-induced asthma model mice. Int Immunopharmacol 2006; 6: 610–5.
Sacerdote P, Martucci C, Vaccani A, Bariselli F, Panerai AE, Colombo A, et al. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J Neuroimmunol 2005; 159: 97–105.
Cher DJ, Mosmann TR . Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol 1987; 138: 3688–94.
Kobayashi K, Kaneda K, Kasama T . Immunopathogenesis of delayed-type hypersensitivity. Microsc Res Tech 2001; 53: 241–5.
Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 2001; 134: 845–52.
Correa F, Hernangómez M, Mestre L, Loría F, Spagnolo A, Docagne F, et al. Anandamide enhances IL-10 production in activated microglia by targeting CB(2) receptors: roles of ERK1/2, JNK, and NF-kappaB. Glia 2010; 58: 135–47.
Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 2006; 49: 67–79.
Acknowledgements
This work was supported by grant NSC98-2923-B-002-001-MY2 from the National Science Council, and grant 99AS-9.2.5-BQ-B1 (2) from the Council of Agriculture, Executive Yuan, Taiwan, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Dz., Hu, Cm., Huang, Ch. et al. Cannabidiol attenuates delayed-type hypersensitivity reactions via suppressing T-cell and macrophage reactivity. Acta Pharmacol Sin 31, 1611–1617 (2010). https://doi.org/10.1038/aps.2010.155
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.155
Keywords
This article is cited by
-
The Non-Psychoactive Plant Cannabinoid, Cannabidiol Affects Cholesterol Metabolism-Related Genes in Microglial Cells
Cellular and Molecular Neurobiology (2011)