Abstract
Aim:
To evaluate the biomechanical properties of thoracic aorta with or without adventitia, and to determine whether there are corresponding changes with hypertension.
Methods:
Normotensive Wistar-Kyoto (WKY) rats and spontaneously hypertensive rats (SHR) at the age of 16 and 32 weeks were used. Thoracic aortic adventitial layer was mechanically separated from thoracic aorta and the adventitia-denuded artery ring was viewed as thoracic media. A load-strain curve was obtained by stretching the ring-shaped intact thoracic aorta or thoracic media with a tensile testing machine. Then, the slope of the load-stain curve at 30%–40% strains was viewed as the elastic stiffness at physiological load, whereas the slope near the breaking point was calculated as maximum stiffness. The maximum load is the load at the breaking point.
Results:
There was no significant difference in elastic stiffness and maximum stiffness of intact thoracic aorta between SHR and age-matched WKY. The elastic stiffness of intact thoracic aorta showed no significant difference from that of thoracic media in WKY and SHR at both ages. In contrast, both maximum stiffness and maximum load were reduced in thoracic media compared with intact thoracic aorta in SHR and WKY at both ages.
Conclusion:
These results indicated that vascular adventitia contributes to maximum stiffness, but not elastic stiffness in both SHR and WKY.
Similar content being viewed by others
Introduction
The mechanical properties of large conduit arteries play an important role in cardiovascular hemodynamics through the buffering of stroke volume and the propagation1. These large arteries, including thoracic aortas, are made up of three layers: intima, media, and adventitia. The medial layer is composed of vascular smooth muscle cells (VSMCs) and extracellular matrix (ECM), such as collagen and elastin. Functionally, ECM influences the passive mechanical properties of the arterial wall whereas VSMCs are related to its active properties2. Endothelial cells in intimal layer regulate vascular tone by releasing various relaxing or constricting factors3, 4. Correspondingly, pathological changes have typically been attributed to detrimental alterations in endothelial function or the medial layer. In contrast, the role of the adventitia has been relegated to that of structural support or protecting the vessels from overstretch5, 6.
Recent studies, however, showed that the adventitia plays an important role in various vascular processes such as atherosclerosis, hypertension and restenosis after balloon injury7, 8. Moreover, the contribution of adventitia to passive mechanical properties of the artery has been paid more and more attention5, 9, 10, 11, and it has been shown that coronary adventitia contribute to vascular biomechanics in terms of shear, circumferential and axial modulus5, 10. Therefore, data on various layers are essential to the understanding of various diseases (such as hypertension, low overload, diabetes) that may remodel the tissue geometry and biomechanical properties of the vascular wall differentially.
Our previous study showed that thoracic adventitial layer contained as much collagen as that of thoracic media, and that collagen was densely distributed in adventitial layer in Sirius Red Staining, indicating that adventitial layer might play a role in the biomechanical characteristics of thoracic aorta12. Importantly, the thoracic medial layer underwent different remodeling from that of thoracic adventitia in spontaneously hypertensive rats (SHR) compared with normotensive Wistar-Kyoto (WKY) rats12. To elucidate the biomechanical contribution of adventitial layer and the possible alteration in hypertensive objectives, we examined the load-stain relationships of the intact thoracic aorta and thoracic media (adventitial layer was removed) in SHR and WKY.
Materials and methods
Animals
Male WKY and SHR at the age of 16- and 32-week were obtained from the Shanghai Institute of Hypertension. The investigation conforms with guidelines for the care and use of laboratory animals as established by Shanghai Institute of Hypertension and the Ethics Committee of Animal Experiments of the Chinese Academy of Sciences.
Blood pressure measurement
Tail-cuff method was used for blood pressure measurement. In brief, the rats were placed in a chamber at 37 °C for 30 min, the tail cuff was connected to a cylinder of compressed air through an arrangement of inlet and outlet valves that permitted inflation and deflation of the cuff at a constant rate. The signals were then recorded with Powerlab digital recording systems (ADInstruments, Sydney, Australia) mounted in a desktop computer.
Biomechanical measurement
The descending thoracic aortas consisting of the entire segment were harvested between the left subclavian and celiac artery branch points13. The peri-adipose tissue was removed after washing with phosphate-buffered saline (PBS) buffer (in mmol/L): NaCl 150, KCl 2.7, Na2HPO4 10, NaH2PO4 2, pH 7.4. Then the adventitia was mechanically separated from media with fine forceps, and considered as the outer layer, while the intima-media is considered as the inner layer (thoracic media). In order to obtain a static load-strain curve, the samples of the thoracic aorta were analyzed by means of a tensile testing machine (Instron 3343, Instron Corp, Norwood, MA). Each ring-shaped sample was mounted on 2 hooks and was stretched at a constant tensile speed of 2 mm/min14, 15, a load-deformation curve was recorded. Strain is defined as the deformation value divided by the original length of the sample. Elastic stiffness in the region of physiological load values was defined as the slope of a straight line drawn between 30% and 40% strain (N/mm), and maximum stiffness is expressed as the slope of the linear steepest part of the load–strain curves up to the point of failure (N/mm)16, 17, 18. In detail, elastic stiffness was expressed as the load difference between 40% and 30% strain (Y axis, N) divided by stain (X axis, the original vascular rings length multiplied by 10%, mm), and maximum stiffness was expressed as the difference between the breaking point and the point with 10% lower stain (Y axis, N) divided by stain (X axis, the original vascular rings length multiplied by 10%, mm). Maximum load was the load at the breaking point. All of elastic stiffness, maximum stiffness and maximum load were corrected by axial vascular length (cm). The contribution of adventitial layer was evaluated by comparing the value of thoracic aorta with or without adventitia5, 9, 11.
Preparation of tissue samples
After rats were anesthetized, they were exsanguinated via a catheter placed in the right auricle while saline was injected into the femoral catheter. When the liquid from the auricle ran clear, the circulatory tract was rinsed with a 4% formaldehyde solution. The animals died very shortly after the formaldehyde infusion was started, the fixation liquid infused for 3 h at a pressure equal to the mean blood pressure (MBP) of each animal. Tissues were embedded in paraffin and transverse sections (5 μm thick) were prepared for Weigert's Elastin Staining, Hematoxylin & Eosin (H&E) Staining, or Masson's Trichrome Staining.
Statistical analysis
Results are expressed as mean±SD. Statistical comparisons were made by paired or unpaired Student t-test. A value of P <0.05 was considered significant.
Results
Body weight and blood pressure
Basic parameters of rats (n = 8 in each group) were summarized in Table 1. Systolic blood pressure (SBP) was higher in 16- and 32-week-old SHR compared with age-matched WKY (P <0.05, Table 1).
Elastic stiffness of intact thoracic aorta and thoracic media
To elucidate the role of adventitial layer on vascular passive mechanical properties, the thoracic adventitial layer was mechanically removed from thoracic aorta of WKY, and the adventitia-removed artery was viewed as thoracic media. It was shown that adventitial removal did not damage external elastic lamella (Figure 1 A&B) and the integrity of slender elastic fiber in the media was well preserved (Figure 1 C&D). Also, adventitial layer contained much collagen in Masson's Trichrome Staining, and there was no collagen staining in adventitial layer in adventitia-denuded vascular wall (Figure 1 E&F).
Weigert's Elastin Staining (A&B), H&E staining (C&D) and Masson's Trichrome Staining (E&F) of intact thoracic aorta (A&C&E) and thoracic media (B&D&F) in 16-week-old WKY. Schematic representation of a typical load-strain curve of intact thoracic aorta (G). The elastic stiffness is the defined as the slope at physiological load corresponding to 30%-40% strain, and the maximum stiffness is defined as the slope of the maximum strain calculated from the breaking point and the point with 10% lower stain. The maximum load is the load at the breaking point.
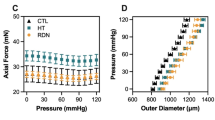
Then, we examined the biomechanical characteristics of thoracic aortas from 16- and 32-week-old SHR and WKY with or without adventitial layers. Samples were stretched by using a tensile machine, after load-strain curves were obtained the elastic stiffness (30%−40% strain) was calculated (Figure 1G). There was no difference in elastic stiffness of intact thoracic aorta (with adventitia) between SHR and WKY at both ages (P >0.05, Table 2). Similarly, in thoracic media (without adventitia), there was no difference in thoracic media between WKY and SHR at both ages (P >0.05, Table 2). Finally, elastic stiffness was not decreased after adventitial layers were removed in SHR and WKY at both ages (P >0.05, Table 2).
Maximum stiffness of intact thoracic aorta and thoracic media
We also determined whether adventitial layer would affect maximum stiffness and whether there are corresponding age-related changes by further stretching the arterial rings to broken, and the slope of the linear steepest part of the load–strain curves up to the point of failure was calculated (Figure 1G). As shown in Table 2&3, the maximum stiffness was approximately 7 times greater than elastic stiffness. There was no difference in maximum stiffness between SHR and WKY at both ages (P>0.05, Table 3). Notably, the maximum stiffness was markedly decreased in thoracic media compared with intact thoracic aorta in WKY and SHR at both ages (P<0.05, Table 3). The maximum load was decreased after adventitial layers were removed (P<0.05, Table 4).
Discussion
This study examined the contribution of thoracic medial and adventitial layers to artery stiffness of thoracic aorta in SHR and WKY at different ages. We found that adventitial layers removal reduced maximum stiffness but not elastic stiffness of thoracic aorta in both SHR and WKY.
In the present study, the thoracic media and adventitia were mechanically separated and the integrity of media was well preserved as shown with morphological investigation in the present study. Then, load-strain curves of intact thoracic aorta and thoracic media were obtained to detect arterial stiffness. The contribution of adventitial layer was estimated by comparing the differences, which has been extensively used in the study of the biomechanical properties of adventitial layer5, 9, 11.
Previous studies showed that mechanical characteristics of thoracic aorta were not changed in SHR compared with WKY2, 19. A study with carotid showed that arterial distensibility was increased in 4, 8 16, 32-week-old SHR and stroke prone SHR (SHR-SP) compared with that in normotensive control animals for similar blood pressure levels, although there was an age-related decrease in arterial distensibility in rats of all groups that was more pronounced in the SHR-SP and SHR compared with that in WKY rats20. Safar showed isobaric aortic pulse wave velocity (PWV) and incremental elastic modulus are significantly increased in old SHR compared with age-matched WKY rats (52 and 78 weeks), but not in young rats (5 or 12 weeks)21, 22. These results indicated that the arterial stiffness was increased in old hypertensive rats but not in young rats. In 2-kidney 1-clip hypertensive rats, carotid arteries were stiffer after 9 and 24 weeks, but no stiffening had occurred at 1 and 5 weeks after renal artery clipping23. In consistent with these above findings, the present study showed that there was no significant difference between SHR and WKY at the age of 16 and 32 week in terms of elastic stiffness and maximum stiffness in intact thoracic aorta, or in thoracic media (without adventitia), which is consistent with our previous finding that elastin : collagen ratio in thoracic media and adventitia was not changed in SHR and WKY, although there is significant medial remodeling in 16- and 32-week old SHR compared with age-matched WKY including increased mass, collagen and elastin content, thoracic adventitial collagen12. These results indicated that elastin : collagen ratio may be the determinant of vascular stiffness24, 25, 26. Namely, collagen abnormity at early hypertension stage does not definitely lead to arterial stiffness increase, although collagen abnormity at later hypertension stage can cause maximum stiffness increase, which is indicative of collagen abnormity of arterial wall in hypertension, diabetes etc20, 27, 28. In addition, vascular component remodeling may occur far before arterial stiffness alteration as shown in this and previous studies. In contrast, the increased distensibility may be caused by the compensation of arteries in response to elevating blood pressure in young objects2, 19, and arterial stiffness increases significantly with age which will be more pronounced in hypertensive objectives than normotensive control.
The medial layer and adventitial layer have different biochemical component and biomechanical characteristics. There are extensive studies on the thoracic media; the function of adventitial layer, however, remains largely unknown. The present study showed that maximum stiffness was reduced after adventitial removal in WKY and SHR at both ages, indicating that adventitial layers play a contributory role in vascular biomechanical properties. This finding is consistent with our previous study showing that there were abundant densely distributed collagen in Sirius Red Staining, and hydroxyproline measurement showed that adventitial layer collagen was comparable to that in the medial layer12. These finding indicated that adventitial layer might be a pharmacological target in the prevention and treatment of cardiovascular diseases. The reduced maximum load after adventitial removal was in line with the traditional view that adventitia protects the vessels from overstretch5. In contrast, elastic stiffness was not markedly reduced after adventitial layer was removed in SHR and WKY, suggesting that adventitial layers do not contribute to passive mechanics at physiological load.
In conclusion, these results demonstrated that thoracic media, but not adventitial layer, contributed to elastic stiffness of thoracic aorta in WKY and SHR, whereas both medial and adventitial layer partially contributed to maximum stiffness of thoracic aorta in WKY and SHR. These results indicated that vascular adventitia contribute to vascular biomechanical properties, and may be involved in pathological process.
Author contribution
Ping-jin GAO and Ding-liang ZHU designed the research, Wei-qing HAN, Jing CHEN and Ling-yun WU conducted the experiments. All five authors contributed to manuscript writing.
References
Benetos A, Laurent S, Asmar RG, Lacolley P . Large artery stiffness in hypertension. J Hypertens Suppl 1997; 15: S89–97.
Bezie Y, Lamaziere JM, Laurent S, Challande P, Cunha RS, Bonnet J, et al. Fibronectin expression and aortic wall elastic modulus in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 1998; 18: 1027–34.
Tang EH, Vanhoutte PM . Prostanoids and reactive oxygen species: team players in endothelium-dependent contractions. Pharmacol Ther 2009; 122: 140–9.
Han WQ, Zhu DL, Wu LY, Chen QZ, Guo SJ, et al. N-acetylcysteine-induced vasodilation involves voltage-gated potassium channels in rat aorta. Life Sci 2009; 84: 732–7.
Lu X, Pandit A, Kassab GS . Biaxial incremental homeostatic elastic moduli of coronary artery: two-layer model. Am J Physiol Heart Circ Physiol 2004; 287: H1663–1669.
Laflamme K, Roberge CJ, Grenier G, Remy-Zolghadri M, Pouliot S, Baker K, et al. Adventitia contribution in vascular tone: insights from adventitia-derived cells in a tissue-engineered human blood vessel. Faseb J 2006; 20: 1245–7.
Li L, Zhu DL, Shen WL, Gao PJ . Increased migration of vascular adventitial fibroblasts from spontaneously hypertensive rats. Hypertens Res 2006; 29: 95–103.
Rey FE, Pagano PJ . The reactive adventitia: fibroblast oxidase in vascular function. Arterioscler Thromb Vasc Biol 2002; 22: 1962–71.
Schulze-Bauer CA, Regitnig P, Holzapfel GA . Mechanics of the human femoral adventitia including the high-pressure response. Am J Physiol Heart Circ Physiol 2002; 282: H2427–2440.
Pandit A, Lu X, Wang C, Kassab GS . Biaxial elastic material properties of porcine coronary media and adventitia. Am J Physiol Heart Circ Physiol 2005; 288: H2581–7.
von Maltzahn WW, Warriyar RG, Keitzer WF . Experimental measurements of elastic properties of media and adventitia of bovine carotid arteries. J Biomech 1984; 17: 839–47.
Han WQ, Wu LY, Zhou HY, Zhang J, Che ZQ, Wu YJ, et al. Changes in the composition of the thoracic aortic wall in spontaneously hypertensive rats treated with losartan or spironolactone. Clin Exp Pharmacol Physiol 2009; 36: 583–8.
Ehrhart LA, Ferrario CM . Collagen metabolism and reversal of aortic medial hypertrophy in spontaneously hypertensive rats treated with methyldopa. Hypertension 1981; 3: 479–84.
Mizutani K, Ikeda K, Kawai Y, Yamori Y . Biomechanical properties and chemical composition of the aorta in genetic hypertensive rats. J Hypertens 1999; 17: 481–7.
Oxlund H . Changes in the biomechanical properties of skin and aorta induced by corticotrophin treatment. Acta Endocrinol (Copenh) 1980; 94: 132–7.
Bruel A, Oxlund H . Changes in biomechanical properties, composition of collagen and elastin, and advanced glycation endproducts of the rat aorta in relation to age. Atherosclerosis 1996; 127: 155–65.
Tham DM, Martin-McNulty B, Wang YX, Da Cunha V, Wilson DW, Athanassious CN, et al. Angiotensin II injures the arterial wall causing increased aortic stiffening in apolipoprotein E-deficient mice. Am J Physiol Regul Integr Comp Physiol 2002; 283: R1442–9.
Chai S, Chai Q, Danielsen CC, Hjorth P, Nyengaard JR, Ledet T, et al. Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circ Res 2005; 96: 583–91.
Cohuet G, Challande P, Osborne-Pellegrin M, Arribas SM, Dominiczak A, Louis H, et al. Mechanical strength of the isolated carotid artery in SHR. Hypertension 2001; 38: 1167–71.
Zanchi A, Brunner HR, Hayoz D . Age-related changes of the mechanical properties of the carotid artery in spontaneously hypertensive rats. J Hypertens 1997; 15: 1415–22.
Safar M, Chamiot-Clerc P, Dagher G, Renaud JF . Pulse pressure, endothelium function, and arterial stiffness in spontaneously hypertensive rats. Hypertension 2001; 38: 1416–21.
Safar M, Laurent P . Pulse pressure and arterial stiffness in rats: comparison with humans. Am J Physiol Heart Circ Physiol 2003; 285: H1363–1369.
Zanchi A, Wiesel P, Aubert JF, Brunner HR, Hayoz D . Time course changes of the mechanical properties of the carotid artery in renal hypertensive rats. Hypertension 1997; 29: 1199–203.
Cantini C, Kieffer P, Corman B, Liminana P, Atkinson J, Lartaud-Idjouadiene I . Aminoguanidine and aortic wall mechanics, structure, and composition in aged rats. Hypertension 2001; 38: 943–8.
Basu P, Sen U, Tyagi N, Tyagi SC . Blood flow interplays with elastin: collagen and MMP: TIMP ratios to maintain healthy vascular structure and function. Vasc Health Risk Manag 2010; 6: 215–28.
Hashimoto S, Yokokura T, Mutai M . Effect of age and dietary composition on aortic collagen to elastin ratio and on lipid metabolism in F-344 rat. Exp Gerontol 1981; 16: 35–9.
Berk BC, Fujiwara K, Lehoux S . ECM remodeling in hypertensive heart disease. J Clin Invest 2007; 117: 568–75.
Pfeiffer BJ, Franklin CL, Hsieh FH, Bank RA, Phillips CL . Alpha 2(I) collagen deficient oim mice have altered biomechanical integrity, collagen content, and collagen crosslinking of their thoracic aorta. Matrix Biol 2005; 24: 451–8.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No 30670832, 30870941, 30871085), National Key Project for Basic Research (No 2006CB503804, 2009CB521905), National High-tech R&D Program (No 2006AA02Z179), grants from the Shanghai Science and Technology Committee (No 08JC1417400, 08DZ2200400, 08410702400, 09540704600) and Shanghai Pujiang Program (08PJ1408000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, Wq., Chen, J., Wu, Ly. et al. Different biomechanical properties of medial and adventitial layers of thoracic aorta in Wistar-Kyoto and spontaneously hypertensive rats. Acta Pharmacol Sin 31, 1319–1323 (2010). https://doi.org/10.1038/aps.2010.121
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.121
Keywords
This article is cited by
-
Eicosapentaenoic acid/docosahexaenoic acid 1:1 ratio improves histological alterations in obese rats with metabolic syndrome
Lipids in Health and Disease (2014)
-
Frontiers of vascular biology and disease research
Acta Pharmacologica Sinica (2010)