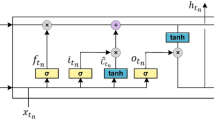
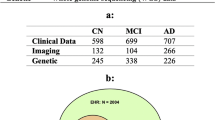
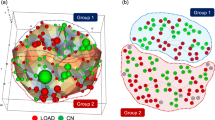
Abstract
Alzheimer’s disease, which is characterized by a continual deterioration of cognitive abilities in older people, is the most common form of dementia. Neuroimaging data, for example, from magnetic resonance imaging and positron emission tomography, enable identification of the structural and functional changes caused by Alzheimer’s disease in the brain. Diagnosing Alzheimer’s disease is critical in medical settings, as it supports early intervention and treatment planning and contributes to expanding our knowledge of the dynamics of Alzheimer’s disease in the brain. Lately, ensemble deep learning has become popular for enhancing the performance and reliability of Alzheimer’s disease diagnosis. These models combine several deep neural networks to increase a prediction’s robustness. Here we revisit key developments of ensemble deep learning, connecting its design—the type of ensemble, its heterogeneity and data modalities—with its application to AD diagnosis using neuroimaging and genetic data. Trends and challenges are discussed thoroughly to assess where our knowledge in this area stands.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Huang, Y. et al. A machine learning approach to brain epigenetic analysis reveals kinases associated with Alzheimer’s disease. Nat. Commun. 12, 4472 (2021).
DeTure, M. A. & Dickson, D. W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 14, 1–18 (2019).
Gaugler, J. et al. Alzheimer’s disease facts and figures. Alzheimers Dementia 18, 700–789 (2022).
Lee, G., Nho, K., Kang, B., Sohn, K.-A. & Kim, D. Predicting Alzheimer’s disease progression using multi-modal deep learning approach. Sci. Rep. 9, 1952 (2019).
Seitz-Holland, J. et al. Major depression, physical health and molecular senescence markers abnormalities. Nat. Mental Health 1, 200–209 (2023).
Scheltens, P. Mild cognitive impairment—amyloid and beyond. Nat. Rev. Neurol. 9, 493–495 (2013).
Lyketsos, C. G. et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 288, 1475–1483 (2002).
Eikelboom, W. S. et al. Neuropsychiatric and cognitive symptoms across the Alzheimer disease clinical spectrum: cross-sectional and longitudinal associations. Neurology 97, e1276–e1287 (2021).
Pinyopornpanish, K. et al. Impact of behavioral and psychological symptoms of Alzheimer’s disease on caregiver outcomes. Sci. Rep. 12, 14138 (2022).
Yu, B., Shan, Y. & Ding, J. A literature review of MRI techniques used to detect amyloid-beta plaques in Alzheimer’s disease patients. Ann. Palliat. Med. 10, 10062–10074 (2021).
Rathore, S., Habes, M., Iftikhar, M. A., Shacklett, A. & Davatzikos, C. A review on neuroimaging-based classification studies and associated feature extraction methods for Alzheimer’s disease and its prodromal stages. NeuroImage 155, 530–548 (2017).
Hedayati, R., Khedmati, M. & Taghipour-Gorjikolaie, M. Deep feature extraction method based on ensemble of convolutional auto encoders: application to Alzheimer’s disease diagnosis. Biomed. Signal Process. Control 66, 102397 (2021).
Pan, D. et al. Early detection of Alzheimer’s disease using magnetic resonance imaging: a novel approach combining convolutional neural networks and ensemble learning. Front. Neurosci. 14, 259 (2020).
Ahmed, S., Kim, B. C., Lee, K. H. & Jung, H. Y., Alzheimer’s Disease Neuroimaging Initiative. Ensemble of ROI-based convolutional neural network classifiers for staging the Alzheimer disease spectrum from magnetic resonance imaging. PLoS ONE 15, e0242712 (2020).
Dietterich, T. G. Ensemble methods in machine learning. In Proc. International Workshop on Multiple Classifier Systems, (eds Kittler, J. & Roli, F.) Vol. 1857, 1–15 (Springer, 2000).
Dong, X., Yu, Z., Cao, W., Shi, Y. & Ma, Q. A survey on ensemble learning. Front. Comput. Sci. 14, 241–258 (2020).
Grewal, J. et al. Application of a neural network whole transcriptome-based pan-cancer method for diagnosis of primary and metastatic cancers. JAMA Netw. Open 2, e192597 (2019).
Karim, M. R., Rahman, A., Jares, J. B., Decker, S. & Beyan, O. A snapshot neural ensemble method for cancer-type prediction based on copy number variations. Neural Comput. Appl. 32, 15281–15299 (2020).
Bartoszewicz, J. M., Seidel, A., Rentzsch, R. & Renard, B. Y. DeePaC: predicting pathogenic potential of novel DNA with reverse-complement neural networks. Bioinformatics 36, 81–89 (2020).
Cao, Z., Pan, X., Yang, Y., Huang, Y. & Shen, H.-B. The lncLocator: a subcellular localization predictor for long non-coding RNAs based on a stacked ensemble classifier. Bioinformatics 34, 2185–2194 (2018).
Zohora, F. T. et al. DeepIso: a deep learning model for peptide feature detection from LC-MS map. Sci. Rep. 9, 17168 (2019).
Singh, J., Hanson, J., Paliwal, K. & Zhou, Y. RNA secondary structure prediction using an ensemble of two-dimensional deep neural networks and transfer learning. Nat. Commun. 10, 5407 (2019).
Polikar, R. in Ensemble Machine Learning: Methods Applications 1–34 (Springer, 2012).
Cao, Y., Geddes, T. A., Yang, J. Y. H. & Yang, P. Ensemble deep learning in bioinformatics. Nat. Mach. Intell. 2, 500–508 (2020).
Ebrahimighahnavieh, M. A., Luo, S. & Chiong, R. Deep learning to detect Alzheimer’s disease from neuroimaging: a systematic literature review. Comput. Methods Programs Biomed. 187, 105242 (2020).
Yang, Y., Lv, H. & Chen, N. A survey on ensemble learning under the era of deep learning. Artif. Intell. Rev. 56, 5545–5589 (2023).
Guo, C., Liu, M. & Lu, M. A dynamic ensemble learning algorithm based on K-means for ICU mortality prediction. Appl. Soft Comput. 103, 107166 (2021).
Bengio, Y., Courville, A. & Vincent, P. Representation learning: a review and new perspectives. IEEE Trans. Pattern Anal. Mach. Intell. 35, 1798–1828 (2013).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Ganaie, M. A., Hu, M., Malik, A., Tanveer, M. & Suganthan, P. N. Ensemble deep learning: a review. Eng. Appl. Artif. Intell. 115, 105151 (2022).
Simonyan, K. & Zisserman, A. Very deep convolutional networks for large-scale image recognition. Preprint at https://arxiv.org/abs/1409.1556 (2014).
Matloob, F. et al. Software defect prediction using ensemble learning: a systematic literature review. IEEE Access 9, 98754–98771 (2021).
Xie, J., Xu, B. & Chuang, Z. Horizontal and vertical ensemble with deep representation for classification. Preprint at https://arxiv.org/abs/1306.2759 (2013).
Laine, S. & Aila, T. Temporal ensembling for semi-supervised learning. International Conference on Learning Representations (ICLR) 2017.
Ciregan, D., Meier, U. & Schmidhuber, J. Multi-column deep neural networks for image classification. In Proc. 2012 IEEE Conference on Computer Vision and Pattern Recognition 3642–3649 (IEEE, 2012).
Grassmann, F. et al. A deep learning algorithm for prediction of age-related eye disease study severity scale for age-related macular degeneration from color fundus photography. Ophthalmology 125, 1410–1420 (2018).
Tabik, S. et al. MNIST-NET10: a heterogeneous deep networks fusion based on the degree of certainty to reach 0.1% error rate. Ensembles overview and proposal. Inf. Fusion 62, 73–80 (2020).
Srivastava, N. et al. Dropout: a simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 15, 1929–1958 (2014).
Wan, L., Zeiler, M., Zhang, S., Cun, Y. L. & Fergus, R. Regularization of neural networks using DropConnect. In Proc. 30th International Conference on Machine Learning Vol. 28-3 (eds Dasgupta, S. & McAllester, D.) 1058–1066 (PMLR, 2013).
Huang, G., Sun, Y., Liu, Z., Sedra, D. & Weinberger, K. Q. Deep networks with stochastic depth. In Proc. European Conference on Computer Vision 646–661 (Springer, 2016).
Singh, S., Hoiem, D. & Forsyth, D. Swapout: Learning an ensemble of deep architectures. In 30th Conf. Neural Information Processing Systems (2016).
Hinton, G., Vinyals, O. & Dean, J. Distilling the knowledge in a neural network. Preprint at https://arxiv.org/abs/1503.02531 (2015).
Smith, L. N., Hand, E. M. & Doster, T. Gradual dropin of layers to train very deep neural networks. In Proc. IEEE Conference on Computer Vision and Pattern Recognition 4763–4771 (IEEE, 2016).
Wolpert, D. H. Stacked generalization. Neural Netw. 5, 241–259 (1992).
Ebrahimi, A., Luo, S. & Chiong, R., Alzheimer’s Disease Neuroimaging Initiative Deep sequence modelling for Alzheimer’s disease detection using MRI. Comput. Biol. Med. 134, 104537 (2021).
Tanveer, M. et al. Classification of Alzheimer’s disease using ensemble of deep neural networks trained through transfer learning. IEEE J. Biomed. Health Informatics 26, 1453–1463 (2022).
Maji, K., Sharma, R., Verma, S. & Goel, T. RVFL classifier based ensemble deep learning for early diagnosis of Alzheimer’s Disease. In Proc. 29th Neural Information Processing Part III 616–626 (Springer, 2023).
Razzak, I. et al. Multiresolutional ensemble PartialNet for Alzheimer detection using magnetic resonance imaging data. Int. J. Intell. Syst. 37, 6613–6630 (2022).
Razzak, I., Naz, S., Alinejad-Rokny, H., Nguyen, T. N. & Khalifa, F. A cascaded mutliresolution ensemble deep learning framework for large scale Alzheimer’s disease detection using brain MRIs. Proc. IEEE/ACM Trans. Comput. Biol. Bioinformatics 1–9 (2022); https://doi.org/10.1109/TCBB.2022.3219032
Wang, R., Li, H., Lan, R., Luo, S. & Luo, X. Hierarchical ensemble learning for Alzheimer’s disease classification. In Proc. 2018 7th International Conference on Digital Home (ICDH) 224–229 (IEEE, 2018).
Zheng, C., Xia, Y., Chen, Y., Yin, X. & Zhang, Y. Early diagnosis of Alzheimer’s disease by ensemble deep learning using FDG-PET. In Proc. International Conference on Intelligent Science and Big Data Engineering 614–622 (Springer, 2018).
Zeng, A. et al. Discovery of genetic biomarkers for Alzheimer’s disease using adaptive convolutional neural networks ensemble and genome-wide association studies. Interdiscip. Sci. Comput. Life Sci. 13, 787–800 (2021).
Sharma, R. et al. Conv-ERVFL: convolutional neural network based ensemble RVFL classifier for Alzheimer’s disease diagnosis. IEEE J. Biomed. Health Informatics 27, 4995–5003 (2023).
Zhang, T. & Shi, M. Multi-modal neuroimaging feature fusion for diagnosis of Alzheimer’s disease. J. Neurosci. Methods 341, 108795 (2020).
Tang, C. et al. CsAGP: detecting Alzheimer’s disease from multimodal images via dual-transformer with cross-attention and graph pooling. J. King Saud Univ. Inf. Sci. 101618 (2023).
Haque, M. N., Noman, N., Berretta, R. & Moscato, P. Heterogeneous ensemble combination search using genetic algorithm for class imbalanced data classification. PLoS ONE 11, e0146116 (2016).
Choi, J. Y. & Lee, B. Combining of multiple deep networks via ensemble generalization loss, based on MRI images, for Alzheimer’s disease classification. IEEE Signal Process. Lett. 27, 206–210 (2020).
Ji, H., Liu, Z., Yan, W. Q. & Klette, R. Early diagnosis of Alzheimer’s disease using deep learning. In Proc. 2nd International Conference on Control and Computer Vision 87–91 (ACM, 2019).
Kang, W. et al. Multi-model and multi-slice ensemble learning architecture based on 2D convolutional neural networks for Alzheimer’s disease diagnosis. Comput. Biol. Med. 136, 104678 (2021).
Sadat, S. U. et al. Alzheimer’s disease detection and classification using transfer learning technique and ensemble on convolutional neural networks. In Proc. 2021 IEEE International Conference on Systems, Man and Cybernetics (SMC) 1478–1481 (IEEE, 2021).
Islam, J. & Zhang, Y. An ensemble of deep convolutional neural networks for Alzheimer’s disease detection and classification. Preprint at https://arxiv.org/abs/1712.01675 (2017).
Jabason, E., Ahmad, M. O. & Swamy, M. Classification of Alzheimer’s disease from MRI data using an ensemble of hybrid deep convolutional neural networks. In Proc. 2019 IEEE 62nd International Midwest Symposium on Circuits and Systems (MWSCAS) 481–484 (IEEE, 2019).
Khanna, M. Multi-level classification of Alzheimer’s disease using DCNN and ensemble deep learning techniques. Signal Image Video Process. 17, 3603–3611 (2023).
Yang, Y., Li, X., Wang, P., Xia, Y. & Ye, Q. Multi-source transfer learning via ensemble approach for initial diagnosis of Alzheimer’s disease. IEEE J. Transl. Eng. Health Med. 8, 1400310 (2020).
Sethuraman, S. K. et al. Predicting Alzheimer’s disease using deep neuro-functional networks with resting-state fMRI. Electronics 12, 1031 (2023).
Giovannetti, A. et al. Deep-MEG: spatiotemporal cnn features and multiband ensemble classification for predicting the early signs of Alzheimer’s disease with magnetoencephalography. Neural Comput. Appl. 33, 14651–14667 (2021).
Ying, Q. et al. Multi-modal data analysis for Alzheimer’s disease diagnosis: an ensemble model using imagery and genetic features. In Proc. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 3586–3591 (IEEE, 2021).
Ismail, W. N., PP, F. R. & Ali, M. A. A meta-heuristic multi-objective optimization method for Alzheimer’s disease detection based on multi-modal data. Mathematics 11, 957 (2023).
Yang, L. et al. Deep learning based multimodal progression modeling for Alzheimer’s disease. Stat. Biopharm. Res 13, 337–343 (2021).
Fang, X., Liu, Z. & Xu, M. Ensemble of deep convolutional neural networks based multi-modality images for Alzheimer’s disease diagnosis. IET Image Process. 14, 318–326 (2020).
Ambastha, A. K., Leong, T.-Y. & Alzheimer’s Disease Neuroimaging Initiative. A deep learning approach to neuroanatomical characterisation of Alzheimer’s disease. In Proc. MEDINFO 2017: Precision Healthcare through Informatics 1249–1249 (IOS Press, 2017).
Suk, H.-I., Lee, S.-W. & Shen, D., Alzheimer’s Disease Neuroimaging Initiative. Deep ensemble learning of sparse regression models for brain disease diagnosis. Med. Image Anal. 37, 101–113 (2017).
Wang, H. et al. Ensemble of 3D densely connected convolutional network for diagnosis of mild cognitive impairment and Alzheimer’s disease. Neurocomputing 333, 145–156 (2019).
Ruiz, J., Mahmud, M., Modasshir, M., Kaiser, M. S. & Alzheimer’s Disease Neuroimaging Initiative. 3D DenseNet ensemble in 4-way classification of Alzheimer’s disease. In Proc. International Conference on Brain Informatics 85–96 (Springer, 2020).
Chen, Y. & Xia, Y. Iterative sparse and deep learning for accurate diagnosis of Alzheimer’s disease. Pattern Recognit. 116, 107944 (2021).
Malik, A. & Tanveer, M. Graph embedded ensemble deep randomized network for diagnosis of Alzheimer’s disease. IEEE/ACM Trans. Comput. Biol. Bioinformatics https://doi.org/10.1109/TCBB.2022.3202707 (2022).
Ganaie, M. A. & Tanveer, M. Ensemble deep random vector functional link network using privileged information for Alzheimer’s disease diagnosis. IEEE/ACM Trans. Comput. Biol. Bioinformatics https://doi.org/10.1109/TCBB.2022.3170351 (2022).
Colbaugh, R., Glass, K. & Gallegos, G. Ensemble transfer learning for Alzheimer’s disease diagnosis. In Proc. 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 3102–3105 (IEEE, 2017).
Lu, D., Popuri, K., Ding, G. W., Balachandar, R. & Beg, M. F. Multimodal and multiscale deep neural networks for the early diagnosis of Alzheimer’s disease using structural MR and FDG-PET images. Sci. Rep. 8, 5697 (2018).
Zhang, J. et al. Multi-modal cross-attention network for Alzheimer’s disease diagnosis with multi-modality data. Comput. Biol. Med. 162, 107050 (2023).
El-Sappagh, S., Abuhmed, T., Islam, S. R. & Kwak, K. S. Multimodal multitask deep learning model for Alzheimer’s disease progression detection based on time series data. Neurocomputing 412, 197–215 (2020).
An, N., Ding, H., Yang, J., Au, R. & Ang, T. F. Deep ensemble learning for Alzheimer’s disease classification. J. Biomed. Informatics 105, 103411 (2020).
Ortiz, A., Munilla, J., Gorriz, J. M. & Ramirez, J. Ensembles of deep learning architectures for the early diagnosis of the Alzheimer’s disease. Int. J. Neural Syst. 26, 1650025 (2016).
Donini, M. et al. Combining heterogeneous data sources for neuroimaging based diagnosis: re-weighting and selecting what is important. Neuroimage 195, 215–231 (2019).
Khanna, S. et al. Using multi-scale genetic, neuroimaging and clinical data for predicting Alzheimer’s disease and reconstruction of relevant biological mechanisms. Sci. Rep. 8, 11173 (2018).
Qiu, S. et al. Multimodal deep learning for Alzheimer’s disease dementia assessment. Nat. Commun. 13, 3404 (2022).
Venugopalan, J., Tong, L., Hassanzadeh, H. R. & Wang, M. D. Multimodal deep learning models for early detection of Alzheimer’s disease stage. Sci. Rep. 11, 3254 (2021).
Buciluǎ, C., Caruana, R. & Niculescu-Mizil, A. Model compression. In Proc. 12th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 535–541 (ACM, 2006).
Cheng, W. X., Suganthan, P. N. & Katuwal, R. Time series classification using diversified ensemble deep random vector functional link and Resnet features. Appl. Soft Comput. 112, 107826 (2021).
Bengio, Y., Lecun, Y. & Hinton, G. Deep learning for AI. Commun. ACM 64, 58–65 (2021).
Li, X. et al. Interpretable deep learning: Interpretation, interpretability, trustworthiness and beyond. Knowl. Inf. Syst. 64, 3197–3234 (2022).
Ali, S. et al. Explainable artificial intelligence (XAI): what we know and what is left to attain trustworthy artificial intelligence. Inf. Fusion 99, 101805 (2023).
Díaz-Rodríguez, N. et al. Connecting the dots in trustworthy artificial intelligence: from AI principles, ethics and key requirements to responsible AI systems and regulation. Inf. Fusion 99, 101896 (2023).
Kadmon, J. & Sompolinsky, H. Optimal architectures in a solvable model of deep networks. In Proc. Advances in Neural Information Processing Systems Vol. 29 (NIPS, 2016).
Liu, W. et al. A survey of deep neural network architectures and their applications. Neurocomputing 234, 11–26 (2017).
Rieke, N. et al. The future of digital health with federated learning. NPJ Digit. Med. 3, 119 (2020).
Zhou, Z.-H. & Feng, J. Deep forest. Natl Sci. Rev. 6, 74–86 (2019).
Rabin, L. A., Smart, C. M. & Amariglio, R. E. Subjective cognitive decline in preclinical Alzheimer’s disease. Annu. Rev. Clin. Psychol. 13, 369–396 (2017).
Dadar, M. et al. Performance comparison of 10 different classification techniques in segmenting white matter hyperintensities in aging. NeuroImage 157, 233–249 (2017).
Acknowledgements
This study was funded by the National Supercomputing Mission under DST and Miety, the Government of India under grant no. DST/NSM/R&D_HPC_Appl/ 2021/03.29 and the Science and Engineering Research Board (SERB), Government of India, under the Ramanujan Fellowship Scheme, grant no. SB/S2/RJN-001/2016. J.D.S. acknowledges funding support from the Spanish Centro para el Desarrollo Tecnológico Industrial (CDTI) through the AI4ES project, as well as from the Department of Education of the Basque Government (consolidated research group MATHMODE, IT1456-22). C.T.L. acknowledges funding support from the Australian Research Council (ARC, DP220100803), Australian National Health and Medical Research Council (NHMRC) Ideas Grant APP2021183, and the UTS Human-Centric AI Centre, sponsored by GrapheneX (2023–2031). We also extend our sincere appreciation to M. A. Ganaie, D. Pranjal and H. Kolukuluru for their help in the initial stages of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanveer, M., Goel, T., Sharma, R. et al. Ensemble deep learning for Alzheimer’s disease characterization and estimation. Nat. Mental Health (2024). https://doi.org/10.1038/s44220-024-00237-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44220-024-00237-x