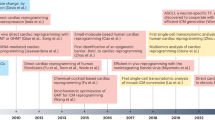
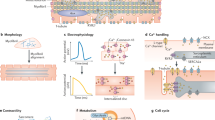
Abstract
Here we aim at providing a concise but comprehensive overview of the perspectives and challenges of heart repair with pluripotent stem cell-derived cardiomyocytes. This Review comes at a time when consensus has been reached about the lack of relevant proliferative capacity of adult mammalian cardiomyocytes and the lack of new heart muscle formation with autologous cell sources. While alternatives to cell-based approaches will be shortly summarized, the focus lies on pluripotent stem cell-derived cardiomyocyte repair, which entered first clinical trials just 2 years ago. In the view of the authors, these early trials are important but have to be viewed as early proof-of-concept trials in humans that will hopefully provide first answers on feasibility, safety and the survival of allogeneic pluripotent stem cell-derived cardiomyocyte in the human heart. Better approaches have to be developed to make this approach clinically applicable.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nabel, E. G. & Braunwald, E. A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 366, 54–63 (2012).
McDonagh, T. A. et al. Group ESCSD. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42, 3599–3726 (2021).
Goldenberg, B. Ueber Atrophie und Hypertrophie der Muskelfasern des Herzens. Arch. Pathol. Anat. Physiol. Klin. Med. 103, 88–130 (1886).
Linzbach, A. J. Heart failure from the point of view of quantitative anatomy. Am. J. Cardiol. 5, 370–382 (1960).
Linzbach, A. J. Hypertrophy, hyperplasia and structural dilatation of the human heart. Adv. Cardiol. 18, 1–14 (1976).
Soonpaa, M. H., Kim, K. K., Pajak, L., Franklin, M. & Field, L. J. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 271, H2183–H2189 (1996).
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Olivetti, G. et al. Apoptosis in the failing human heart. N. Engl. J. Med. 336, 1131–1141 (1997).
Quaini, F. et al. Chimerism of the transplanted heart. N. Engl. J. Med. 346, 5–15 (2002).
Orlic, D. et al. Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 (2001).
Jeyaraman, M. M. et al. Autologous bone marrow stem cell therapy in patients with st-elevation myocardial infarction: a systematic review and meta-analysis. Can. J. Cardiol. 33, 1611–1623 (2017).
Blau, H. M., Brazelton, T. R. & Weimann, J. M. The evolving concept of a stem cell: entity or function? Cell 105, 829–841 (2001).
Dimmeler, S., Zeiher, A. M. & Schneider, M. D. Unchain my heart: the scientific foundations of cardiac repair. J. Clin. Invest. 115, 572–583 (2005).
Beltrami, A. P. et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003).
Oh, H. et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl Acad. Sci. USA 100, 12313–12318 (2003).
Laugwitz, K. L. et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 433, 647–653 (2005).
Planat-Benard, V. et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ. Res. 94, 223–229 (2004).
Condorelli, G. et al. Cardiomyocytes induce endothelial cells to trans-differentiate into cardiac muscle: Implications for myocardium regeneration. Proc. Natl Acad. Sci. USA 98, 10733–10738 (2001).
Li, Y. et al. Genetic lineage tracing of nonmyocyte population by dual recombinases. Circulation 138, 793–805 (2018).
Murry, C. E. et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428, 664–668 (2004).
Nygren, J. M. et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 10, 494–501 (2004).
Laflamme, M. A., Myerson, D., Saffitz, J. E. & Murry, C. E. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ. Res. 90, 634–640 (2002).
Hocht-Zeisberg, E. et al. Cellular repopulation of myocardial infarction in patients with sex-mismatched heart transplantation. Eur. Heart J. 25, 749–758 (2004).
Partners HealthCare and Brigham and Women’s Hospital agree to pay $10 million to resolve research fraud allegations. US Attorney’s Office District of Massachusetts https://www.justice.gov/usao-ma/pr/partners-healthcare-and-brigham-and-women-s-hospital-agree-pay-10-million-resolve (2017).
Eschenhagen, T. et al. Cardiomyocyte regeneration: a consensus statement. Circulation 136, 680–686 (2017).
Senyo, S. E. et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493, 433–436 (2013).
Vagnozzi, R. J. et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 577, 405–409 (2020).
Sadek, H. & Olson, E. N. Toward the goal of human heart regeneration. Cell Stem Cell 26, 7–16 (2020).
Giacca, M. Fulfilling the promise of rna therapies for cardiac repair and regeneration. Stem Cells Transl. Med. 12, 527–535 (2023).
Yamada, Y., Sadahiro, T. & Ieda, M. Development of direct cardiac reprogramming for clinical applications. J. Mol. Cell Cardiol. 178, 1–8 (2023).
Soonpaa, M. H., Koh, G. Y., Klug, M. G. & Field, L. J. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science 264, 98–101 (1994).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Kehat, I. et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest. 108, 407–414 (2001).
Kattman, S. J. et al. Stage-specific optimization of activin/nodal and bmp signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 8, 228–240 (2011).
Burridge, P. W. & Zambidis, E. T. Highly efficient directed differentiation of human induced pluripotent stem cells into cardiomyocytes. Methods Mol. Biol. 997, 149–161 (2013).
Breckwoldt, K. et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat. Protoc. 12, 1177–1197 (2017).
Halloin, C., Coffee, M., Manstein, F. & Zweigerdt, R. Production of cardiomyocytes from human pluripotent stem cells by bioreactor technologies. Methods Mol. Biol. 1994, 55–70 (2019).
Buikema, J. W. et al. Wnt activation and reduced cell–cell contact synergistically induce massive expansion of functional human iPSC-derived cardiomyocytes. Cell Stem Cell 27, 50–63 (2020).
Eschenhagen, T., Ridders, K. & Weinberger, F. How to repair a broken heart with pluripotent stem cell-derived cardiomyocytes. J Mol Cell Cardiol. 163, 106–117 (2022).
Weinberger, F. & Eschenhagen, T. Cardiac regeneration: new hope for an old dream. Annu. Rev. Physiol. 83, 59–81 (2021).
Shiba, Y. et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391 (2016).
Chong, J. J. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014).
Liu, Y. W. et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 36, 597–605 (2018).
Querdel, E. et al. Human engineered heart tissue patches remuscularize the injured heart in a dose-dependent manner. Circulation 143, 1991–2006 (2021).
Riegler, J. et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 117, 720–730 (2015).
Laflamme, M. A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 25, 1015–1024 (2007).
Weinberger, F. et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med. 8, 363ra148 (2016).
Bergmann, O. et al. Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575 (2015).
Lou, X. et al. N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts. Cardiovasc. Res. 116, 671–685 (2020).
Kobayashi, H. et al. Intracoronary transplantation of pluripotent stem cell-derived cardiomyocytes: inefficient procedure for cardiac regeneration. J. Mol. Cell Cardiol. 174, 77–87 (2023).
Li, J. et al. All roads lead to rome (the heart): cell retention and outcomes from various delivery routes of cell therapy products to the heart. J. Am. Heart Assoc. 10, e020402 (2021).
Dow, J., Simkhovich, B. Z., Kedes, L. & Kloner, R. A. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc. Res. 67, 301–307 (2005).
Harris, N. R. et al. The ebb and flow of cardiac lymphatics: a tidal wave of new discoveries. Physiol. Rev. 103, 391–432 (2023).
Kawaguchi, S. et al. Intramyocardial transplantation of human ips cell-derived cardiac spheroids improves cardiac function in heart failure animals. JACC Basic Transl. Sci. 6, 239–254 (2021).
El-Nachef, D. et al. Engrafted human induced pluripotent stem cell-derived cardiomyocytes undergo clonal expansion in vivo. Circulation 143, 1635–1638 (2021).
Laflamme, M. A. et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am. J. Pathol. 167, 663–671 (2005).
Fernandes, S. et al. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J. Mol. Cell Cardiol. 49, 941–949 (2010).
Eschenhagen, T., Mummery, C. & Knollmann, B. C. Modelling sarcomeric cardiomyopathies in the dish: from human heart samples to iPSC cardiomyocytes. Cardiovasc. Res. 105, 424–438 (2015).
Mitsutake, Y. et al. Improvement of local cell delivery using helix transendocardial delivery catheter in a porcine heart. Int. Heart J. 58, 435–440 (2017).
Nakamura, K. et al. Pharmacologic therapy for engraftment arrhythmia induced by transplantation of human cardiomyocytes. Stem Cell Rep. 16, 2473–2487 (2021).
Nakajima, K. et al. Gelatin hydrogel enhances the engraftment of transplanted cardiomyocytes and angiogenesis to ameliorate cardiac function after myocardial infarction. PLoS ONE 10, e0133308 (2015).
Furuta, A. et al. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ. Res. 98, 705–712 (2006).
Kawamura, M. et al. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation 128, S87–S94 (2013).
Zimmermann, W. H. et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 12, 452–458 (2006).
Zhu, W., Zhao, M., Mattapally, S., Chen, S. & Zhang, J. Ccnd2 overexpression enhances the regenerative potency of human induced pluripotent stem cell-derived cardiomyocytes: remuscularization of injured ventricle. Circ. Res. 122, 88–96 (2018).
Zhao, M. et al. Cyclin d2 overexpression enhances the efficacy of human induced pluripotent stem cell-derived cardiomyocytes for myocardial repair in a swine model of myocardial infarction. Circulation 144, 210–228 (2021).
Gabisonia, K. et al. Microrna therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 569, 418–422 (2019).
Gerbin, K. A., Mitzelfelt, K. A., Guan, X., Martinson, A. M. & Murry, C. E. Delta-1 functionalized hydrogel promotes hESC-cardiomyocyte graft proliferation and maintains heart function post-injury. Mol. Ther. Methods Clin. Dev. 17, 986–998 (2020).
Shimizu, T. et al. Long-term survival and growth of pulsatile myocardial tissue grafts engineered by the layering of cardiomyocyte sheets. Tissue Eng. 12, 499–507 (2006).
von Bibra, C. et al. Human engineered heart tissue transplantation in a guinea pig chronic injury model. J. Mol. Cell Cardiol. 166, 1–10 (2022).
Munarin, F., Kant, R. J., Rupert, C. E., Khoo, A. & Coulombe, K. L. K. Engineered human myocardium with local release of angiogenic proteins improves vascularization and cardiac function in injured rat hearts. Biomaterials 251, 120033 (2020).
Ai, X. et al. Transient secretion of VEGF protein from transplanted hiPSC-CMs enhances engraftment and improves rat heart function post MI. Mol. Ther. 31, 211–229 (2023).
Cheng, Y. Y. et al. Metabolic changes associated with cardiomyocyte dedifferentiation enable adult mammalian cardiac regeneration. Circulation 146, 1950–1967 (2022).
Sun, X. et al. Transplanted microvessels improve pluripotent stem cell-derived cardiomyocyte engraftment and cardiac function after infarction in rats. Sci. Transl. Med. 12, eaax2992 (2020).
von Bibra, C. et al. Immature human engineered heart tissues engraft in a guinea pig chronic injury model. Dis. Model Mech. 16, 049834 (2023).
Dhahri, W. et al. In vitro matured human pluripotent stem cell-derived cardiomyocytes form grafts with enhanced structure and function in injured hearts. Circulation 145, 1412–1426 (2022).
Poch, C. M. et al. Migratory and anti-fibrotic programmes define the regenerative potential of human cardiac progenitors. Nat. Cell Biol. 24, 659–671 (2022).
Ottaviani, D., Ter Huurne, M., Elliott, D. A., Bellin, M. & Mummery, C. L. Maturing differentiated human pluripotent stem cells in vitro: methods and challenges. Development https://doi.org/10.1242/dev.201103 (2023).
Shiba, Y. et al. Electrical integration of human embryonic stem cell-derived cardiomyocytes in a guinea pig chronic infarct model. J. Cardiovasc. Pharmacol. Ther. 19, 368–381 (2014).
Gao, L. et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 137, 1712–1730 (2018).
Zhu, K. et al. Lack of remuscularization following transplantation of human embryonic stem cell-derived cardiovascular progenitor cells in infarcted nonhuman primates. Circ. Res. 122, 958–969 (2018).
Shiba, Y. et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325 (2012).
Shadrin, I. Y. et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 8, 1825 (2017).
Studemann, T. et al. Contractile force of transplanted cardiomyocytes actively supports heart function after injury. Circulation 146, 1159–1169 (2022).
Pecha, S. et al. Human iPS cell-derived engineered heart tissue does not affect ventricular arrhythmias in a guinea pig cryo-injury model. Sci. Rep. 9, 9831 (2019).
Romagnuolo, R. et al. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Rep. 12, 967–981 (2019).
Selvakumar, D. et al. Cellular heterogeneity of pluripotent stem cell-derived cardiomyocyte grafts is mechanistically linked to treatable arrhythmias. Nat. Cardiovasc. Res. 3, 145–165 (2024).
Caspi, O. et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J. Am. Coll. Cardiol. 50, 1884–1893 (2007).
Blin, G. et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J. Clin. Invest. 120, 1125–1139 (2010).
Itakura, G. et al. Fail-safe system against potential tumorigenicity after transplantation of ipsc derivatives. Stem Cell Rep. 8, 673–684 (2017).
de Luzy, I. R. et al. Human stem cells harboring a suicide gene improve the safety and standardisation of neural transplants in parkinsonian rats. Nat. Commun. 12, 3275 (2021).
Meissner, T. B., Schulze, H. S. & Dale, S. M. Immune editing: overcoming immune barriers in stem cell transplantation. Curr. Stem Cell Rep. 8, 206–218 (2022).
Simpson, A., Hewitt, A. W. & Fairfax, K. A. Universal cell donor lines: a review of the current research. Stem Cell Rep. https://doi.org/10.1016/j.stemcr.2023.09.010 (2023).
Hu, X. et al. Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat. Biotechnol. 42, 413–423 (2023).
Eschenhagen, T. & Weinberger, F. Heart repair with myocytes. Circ. Res. 124, 843–845 (2019).
Garreta, E., Sanchez, S., Lajara, J., Montserrat, N. & Belmonte, J. C. I. Roadblocks in the path of IPSC to the clinic. Curr. Transplant Rep. 5, 14–18 (2018).
Bogomiakova, M. E. et al. iPSC-derived cells lack immune tolerance to autologous NK-cells due to imbalance in ligands for activating and inhibitory NK-cell receptors. Stem Cell Res. Ther. 14, 77 (2023).
Deuse, T. et al. De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat. Biotechnol. 37, 1137–1144 (2019).
Schweitzer, J. S. et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 382, 1926–1932 (2020).
Acknowledgements
Our work on this topic was supported by a Late Translational Research Grant from the German Centre for Cardiovascular Research (DZHK), (81×2710153 to T.E.), the European Research Council (ERC-AG IndivuHeart to T.E.), the German Research Foundation (DFG, WE5620/3-1 to F.W. and T.E.) and the Werner Otto Stiftung (F.W.). Additionally, we have received funding from the European Union’s Horizon 2020 research and innovation program (874764 to T.E.) and the European Union’s Horizon 2020 FetOpen RIA (964800; to F.W.).
Author information
Authors and Affiliations
Contributions
T.E. drafted the manuscript. F.W. provided substantial contributions to its content. T.E. and F.W. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
T.E. and F.W. contribute in a structured partnership between Evotec AG and the University Medical Center Hamburg-Eppendorf (UKE) to originate a pluripotent stem cell-based remuscularization approach. They have no financial interest and did not obtain consultations fees.
Peer review
Peer review information
Nature Cardiovascular Research thanks Mauro Giacca and Richard Lee for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eschenhagen, T., Weinberger, F. Challenges and perspectives of heart repair with pluripotent stem cell-derived cardiomyocytes. Nat Cardiovasc Res 3, 515–524 (2024). https://doi.org/10.1038/s44161-024-00472-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-024-00472-6