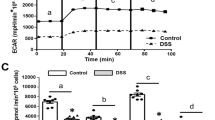
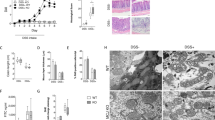
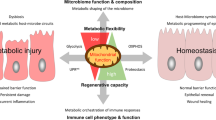
Abstract
Mitochondria are dynamic organelles that function in cellular energy metabolism, intracellular and extracellular signalling, cellular fate and stress responses. Mitochondria of the intestinal epithelium, the cellular interface between self and enteric microbiota, have emerged as crucial in intestinal health. Mitochondrial dysfunction occurs in gastrointestinal diseases, including inflammatory bowel diseases and colorectal cancer. In this Review, we provide an overview of the current understanding of intestinal epithelial cell mitochondrial metabolism, function and signalling to affect tissue homeostasis, including gut microbiota composition. We also discuss mitochondrial-targeted therapeutics for inflammatory bowel diseases and colorectal cancer and the evolving concept of mitochondrial impairment as a consequence versus initiator of the disease.
Key points
-
Mitochondrial health is vital for epithelial barrier function, comprising paracellular junctions, secreted extracellular barrier (mucus and antimicrobial peptides) and the commensal microbiota that is instrumental in inducing immune tolerance.
-
As signalling organelles, the mitochondria produce factors (mitochondrial reactive oxygen species and released mitochondrial DNA) that perpetuate tissue damage and pathological responses.
-
Mutations in genes involved in mitochondrial function and quality control are associated with inflammatory bowel diseases; inflammatory insults drive mitochondrial dysfunction in inflammatory bowel diseases.
-
Mitochondrial dysfunction in intestinal epithelial cells inhibits oxidative phosphorylation, raising oxygen levels, disrupting mucosal oxygen gradient and causing loss of anaerobes that fuel host epithelium.
-
Mitochondrial dysfunction is associated with alterations to cell types proposed to initiate colorectal cancer tumorigenesis in the presence of chronic insult; in established colorectal cancer, mitochondrial metabolism fuels cancer growth.
-
Mitochondrial-targeted therapeutics could provide a dual approach with current treatments in particular subtypes of patients based on genetic mutations, metabolism or biomarkers of mitochondrial stress.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Liu, C. Y., Cham, C. M. & Chang, E. B. Epithelial wound healing in inflammatory bowel diseases: the next therapeutic frontier. Transl. Res. 236, 35–51 (2021).
Alison, M. R. The cellular origins of cancer with particular reference to the gastrointestinal tract. Int. J. Exp. Pathol. 101, 132–151 (2020).
Lee, H., Jeon, J. H. & Kim, E. S. Mitochondrial dysfunctions in T cells: focus on inflammatory bowel disease. Front. Immunol. 14, 1219422 (2023).
Liu, W. et al. Association between oxidative stress, mitochondrial function of peripheral blood mononuclear cells and gastrointestinal cancers. J. Transl. Med. 21, 107 (2023).
Rausser, S. et al. Mitochondrial phenotypes in purified human immune cell subtypes and cell mixtures. eLife 10, e70899 (2021).
Agrawal, M., Allin, K. H., Petralia, F., Colombel, J. F. & Jess, T. Multiomics to elucidate inflammatory bowel disease risk factors and pathways. Nat. Rev. Gastroenterol. Hepatol. 19, 399–409 (2022).
Furey, T. S., Sethupathy, P. & Sheikh, S. Z. Redefining the IBDs using genome-scale molecular phenotyping. Nat. Rev. Gastroenterol. Hepatol. 16, 296–311 (2019).
Raine, T. & Danese, S. Breaking through the therapeutic ceiling: what will it take? Gastroenterology 162, 1507–1511 (2022).
Kotla, N. G. & Rochev, Y. IBD disease-modifying therapies: insights from emerging therapeutics. Trends Mol. Med. 29, 241–253 (2023).
Aldars-Garcia, L., Gisbert, J. P. & Chaparro, M. Metabolomics insights into inflammatory bowel disease: a comprehensive review. Pharmaceuticals 14, 1190 (2021).
Haberman, Y. et al. Ulcerative colitis mucosal transcriptomes reveal mitochondriopathy and personalized mechanisms underlying disease severity and treatment response. Nat. Commun. 10, 38 (2019).
Kugathasan, S. et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 389, 1710–1718 (2017).
Hsieh, S. Y. et al. Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics 6, 5322–5331 (2006).
Mottawea, W. et al. Altered intestinal microbiota–host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun. 7, 13419 (2016).
Alula, K. M. et al. Targeting mitochondrial damage as a therapeutic for ileal Crohn’s disease. Cells 10, 1349 (2021).
Delpre, G., Avidor, I., Steinherz, R., Kadish, U. & Ben-Bassat, M. Ultrastructural abnormalities in endoscopically and histologically normal and involved colon in ulcerative colitis. Am. J. Gastroenterol. 84, 1038–1046 (1989).
O’Morain, C., Smethurst, P., Levi, J. & Peters, T. J. Subcellular fractionation of rectal biopsy homogenates from patients with inflammatory bowel disease. Scand. J. Gastroenterol. 20, 209–214 (1985).
Soderholm, J. D. et al. Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn’s disease. Gut 50, 307–313 (2002).
Sifroni, K. G. et al. Mitochondrial respiratory chain in the colonic mucosal of patients with ulcerative colitis. Mol. Cell. Biochem. 342, 111–115 (2010).
Santhanam, S. et al. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm. Bowel Dis. 18, 2158–2168 (2012).
Santhanam, S., Venkatraman, A. & Ramakrishna, B. S. Impairment of mitochondrial acetoacetyl CoA thiolase activity in the colonic mucosa of patients with ulcerative colitis. Gut 56, 1543–1549 (2007).
Jans, D. & Cleynen, I. The genetics of non-monogenic IBD. Hum. Genet. 142, 669–682 (2023).
Liu, J. Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47, 979–986 (2015).
Ho, G. T. et al. MDR1 deficiency impairs mitochondrial homeostasis and promotes intestinal inflammation. Mucosal Immunol. 11, 120–130 (2018).
Ozsoy, M. et al. Role of energy metabolism and mitochondrial function in inflammatory bowel disease. Inflamm. Bowel Dis. 28, 1443–1450 (2022).
Balasubramanian, I. & Gao, N. From sensing to shaping microbiota: insights into the role of NOD2 in intestinal homeostasis and progression of Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G7–G13 (2017).
Saxena, A., Lopes, F., Poon, K. K. H. & McKay, D. M. Absence of the NOD2 protein renders epithelia more susceptible to barrier dysfunction due to mitochondrial dysfunction. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G26–G38 (2017).
Alula, K. M. & Theiss, A. L. Autophagy in Crohn’s disease: converging on dysfunctional innate immunity. Cells 12, 1779 (2023).
Hudson, G., Gomez-Duran, A., Wilson, I. J. & Chinnery, P. F. Recent mitochondrial DNA mutations increase the risk of developing common late-onset human diseases. PLoS Genet. 10, e1004369 (2014).
Rosa, A. et al. Ulcerative colitis is under dual (mitochondrial and nuclear) genetic control. Inflamm. Bowel Dis. 22, 774–781 (2016).
Baker, K. T. et al. Mitochondrial DNA mutations are associated with ulcerative colitis preneoplasia but tend to be negatively selected in cancer. Mol. Cancer Res. 17, 488–498 (2019).
Pacheu-Grau, D., Rucktaschel, R. & Deckers, M. Mitochondrial dysfunction and its role in tissue-specific cellular stress. Cell Stress 2, 184–199 (2018).
Finsterer, J. & Frank, M. Gastrointestinal manifestations of mitochondrial disorders: a systematic review. Ther. Adv. Gastroenterol. 10, 142–154 (2017).
Gusic, M. & Prokisch, H. Genetic basis of mitochondrial diseases. FEBS Lett. 595, 1132–1158 (2021).
Lee, M. & Chang, E. B. Inflammatory bowel diseases (IBD) and the microbiome — searching the crime scene for clues. Gastroenterology 160, 524–537 (2021).
Ogino, S. et al. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut 67, 1168–1180 (2018).
Hirose, Y. & Taniguchi, K. Intratumoral metabolic heterogeneity of colorectal cancer. Am. J. Physiol. Cell Physiol. 35, C1073–C1084 (2023).
Nguyen, H. T. & Duong, H. Q. The molecular characteristics of colorectal cancer: implications for diagnosis and therapy. Oncol. Lett. 16, 9–18 (2018).
Zhao, R. Z., Jiang, S., Zhang, L. & Yu, Z. B. Mitochondrial electron transport chain, ROS generation and uncoupling (review). Int. J. Mol. Med. 44, 3–15 (2019).
Alcazar-Fabra, M., Navas, P. & Brea-Calvo, G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta 1857, 1073–1078 (2016).
Chandel, N. S. Navigating Metabolism (Cold Spring Harbor Laboratory Press, 2015).
Helander, H. F. & Fandriks, L. Surface area of the digestive tract — revisited. Scand. J. Gastroenterol. 49, 681–689 (2014).
Beumer, J. & Clevers, H. Cell fate specification and differentiation in the adult mammalian intestine. Nat. Rev. Mol. Cell Biol. 22, 39–53 (2021).
Gehart, H. & Clevers, H. Tales from the crypt: new insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 16, 19–34 (2019).
Rodriguez-Colman, M. J. et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543, 424–427 (2017).
Beyaz, S. et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53 (2016).
Mihaylova, M. M. et al. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell 22, 769–778.e4 (2018).
Chen, L. et al. HNF4 regulates fatty acid oxidation and is required for renewal of intestinal stem cells in mice. Gastroenterology 158, 985–999.e9 (2020).
Stine, R. R. et al. PRDM16 maintains homeostasis of the intestinal epithelium by controlling region-specific metabolism. Cell Stem Cell 25, 830–845.e8 (2019).
Schell, J. C. et al. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat. Cell Biol. 19, 1027–1036 (2017).
Jackson, D. N. et al. Mitochondrial dysfunction during loss of prohibitin 1 triggers Paneth cell defects and ileitis. Gut 69, 1928–1938 (2020).
Khaloian, S. et al. Mitochondrial impairment drives intestinal stem cell transition into dysfunctional Paneth cells predicting Crohn’s disease recurrence. Gut 69, 1939–1951 (2020).
Ludikhuize, M. C. et al. Mitochondria define intestinal stem cell differentiation downstream of a FOXO/Notch axis. Cell Metab. 32, 889–900.e7 (2020).
Stringari, C. et al. Metabolic trajectory of cellular differentiation in small intestine by phasor fluorescence lifetime microscopy of NADH. Sci. Rep. 2, 568 (2012).
Byndloss, M. X. et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575 (2017).
Singhal, R. & Shah, Y. M. Oxygen battle in the gut: hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 295, 10493–10505 (2020).
Verzi, M. P. & Shivdasani, R. A. Epigenetic regulation of intestinal stem cell differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 319, G189–G196 (2020).
Jadhav, U. et al. Acquired tissue-specific promoter bivalency is a basis for PRC2 necessity in adult cells. Cell 165, 1389–1400 (2016).
de Sousa, E. M. F. & de Sauvage, F. J. Cellular plasticity in intestinal homeostasis and disease. Cell Stem Cell 24, 54–64 (2019).
Jones, J. C. et al. Cellular plasticity of Defa4(Cre)-expressing Paneth cells in response to notch activation and intestinal injury. Cell. Mol. Gastroenterol. Hepatol. 7, 533–554 (2019).
Tetteh, P. W. et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18, 203–213 (2016).
Westphalen, C. B. et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Invest. 124, 1283–1295 (2014).
Yu, S. et al. Paneth cell multipotency induced by notch activation following injury. Cell Stem Cell 23, 46–59.e5 (2018).
Jadhav, U. et al. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell 21, 65–77.e5 (2017).
Li, Y. et al. ROS signaling-induced mitochondrial Sgk1 expression regulates epithelial cell renewal. Proc. Natl Acad. Sci. USA 120, e2216310120 (2023).
Fan, Y. Y. et al. A bioassay to measure energy metabolism in mouse colonic crypts, organoids, and sorted stem cells. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G1–G9 (2015).
Huang, G. et al. GRIM-19, a cell death regulatory protein, is essential for assembly and function of mitochondrial complex I. Mol. Cell. Biol. 24, 8447–8456 (2004).
Liu, W. et al. Olfactomedin 4 deletion improves male mouse glucose intolerance and insulin resistance induced by a high-fat diet. Endocrinology 159, 3235–3244 (2018).
Taman, H. et al. Genome-wide DNA methylation in treatment-naive ulcerative colitis. J. Crohns Colitis 12, 1338–1347 (2018).
van der Flier, L. G., Haegebarth, A., Stange, D. E., van de Wetering, M. & Clevers, H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 137, 15–17 (2009).
Turrens, J. F. Mitochondrial formation of reactive oxygen species. J. Physiol. 552, 335–344 (2003).
Meyer, J. N., Leuthner, T. C. & Luz, A. L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 391, 42–53 (2017).
Ul Fatima, N. & Ananthanarayanan, V. Mitochondrial movers and shapers: recent insights into regulators of fission, fusion and transport. Curr. Opin. Cell Biol. 80, 102150 (2023).
Formentini, L. et al. Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell Rep. 19, 1202–1213 (2017).
Urbauer, E., Rath, E. & Haller, D. Mitochondrial metabolism in the intestinal stem cell niche — sensing and signaling in health and disease. Front. Cell Dev. Biol. 8, 602814 (2020).
Brand, M. D. et al. Suppressors of superoxide-H2O2 production at site IQ of mitochondrial complex I protect against stem cell hyperplasia and ischemia–reperfusion injury. Cell Metab. 24, 582–592 (2016).
Levy, A. et al. Innate immune receptor NOD2 mediates LGR5(+) intestinal stem cell protection against ROS cytotoxicity via mitophagy stimulation. Proc. Natl Acad. Sci. USA 117, 1994–2003 (2020).
Swanson, P. A. II et al. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc. Natl Acad. Sci. USA 108, 8803–8808 (2011).
Bhattacharyya, A., Chattopadhyay, R., Mitra, S. & Crowe, S. E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94, 329–354 (2014).
Esteras, N. & Abramov, A. Y. Nrf2 as a regulator of mitochondrial function: energy metabolism and beyond. Free Radic. Biol. Med. 189, 136–153 (2022).
Gureev, A. P., Shaforostova, E. A. & Popov, V. N. Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1alpha signaling pathways. Front. Genet. 10, 435 (2019).
Scarpulla, R. C. Nuclear control of respiratory gene expression in mammalian cells. J. Cell. Biochem. 97, 673–683 (2006).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Walker, B. R. & Moraes, C. T. Nuclear–mitochondrial interactions. Biomolecules 12, 427 (2022).
Minocherhomji, S., Tollefsbol, T. O. & Singh, K. K. Mitochondrial regulation of epigenetics and its role in human diseases. Epigenetics 7, 326–334 (2012).
Santos, J. H. Mitochondria signaling to the epigenome: a novel role for an old organelle. Free Radic. Biol. Med. 170, 59–69 (2021).
Matilainen, O., Quiros, P. M. & Auwerx, J. Mitochondria and epigenetics — crosstalk in homeostasis and stress. Trends Cell Biol. 27, 453–463 (2017).
Moore, L. D., Le, T. & Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 38, 23–38 (2013).
Stoccoro, A. & Coppede, F. Mitochondrial DNA methylation and human diseases. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22094594 (2021).
Howell, K. J. et al. DNA methylation and transcription patterns in intestinal epithelial cells from pediatric patients with inflammatory bowel diseases differentiate disease subtypes and associate with outcome. Gastroenterology 154, 585–598 (2018).
Kelly, D. et al. Microbiota-sensitive epigenetic signature predicts inflammation in Crohn’s disease. JCI Insight 3, e122104 (2018).
Kraiczy, J. et al. Assessing DNA methylation in the developing human intestinal epithelium: potential link to inflammatory bowel disease. Mucosal Immunol. 9, 647–658 (2016).
Mokry, M. et al. Many inflammatory bowel disease risk loci include regions that regulate gene expression in immune cells and the intestinal epithelium. Gastroenterology 146, 1040–1047 (2014).
Tsaprouni, L. G., Ito, K., Powell, J. J., Adcock, I. M. & Punchard, N. Differential patterns of histone acetylation in inflammatory bowel diseases. J. Inflamm. 8, 1 (2011).
Heimerl, S. et al. Alterations in intestinal fatty acid metabolism in inflammatory bowel disease. Biochim. Biophys. Acta 1762, 341–350 (2006).
Roediger, W. E. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet 2, 712–715 (1980).
GBD Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30 (2020).
Johnston, R. D. & Logan, R. F. What is the peak age for onset of IBD? Inflamm. Bowel Dis. 14, S4–S5 (2008).
Odenwald, M. A. & Turner, J. R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 14, 9–21 (2017).
Wells, J. M. et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G171–G193 (2017).
Kayama, H., Okumura, R. & Takeda, K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu. Rev. Immunol. 38, 23–48 (2020).
Saint-Georges-Chaumet, Y. & Edeas, M. Microbiota–mitochondria inter-talk: consequence for microbiota–host interaction. Pathog. Dis. 74, ftv096 (2016).
Chang, J. T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 383, 2652–2664 (2020).
Ijssennagger, N., van der Meer, R. & van Mil, S. W. C. Sulfide as a mucus barrier-breaker in inflammatory bowel disease? Trends Mol. Med. 22, 190–199 (2016).
Sargent, A. L. et al. Quantitatively assessing the respiratory burst in innate immune cells. Methods Mol. Biol. 2614, 47–70 (2023).
Bourgonje, A. R. et al. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol. Med. 26, 1034–1046 (2020).
Ghezzi, D. & Zeviani, M. Assembly factors of human mitochondrial respiratory chain complexes: physiology and pathophysiology. Adv. Exp. Med. Biol. 748, 65–106 (2012).
Bar, F. et al. Mitochondrial gene polymorphisms that protect mice from colitis. Gastroenterology 145, 1055–1063.e3 (2013).
Cunningham, K. E. et al. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1alpha) protects against experimental murine colitis. J. Biol. Chem. 291, 10184–10200 (2016).
Han, J., Yu, C., Souza, R. F. & Theiss, A. L. Prohibitin 1 modulates mitochondrial function of Stat3. Cell Signal. 26, 2086–2095 (2014).
Lopez-Armada, M. J., Riveiro-Naveira, R. R., Vaamonde-Garcia, C. & Valcarcel-Ares, M. N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13, 106–118 (2013).
Biniecka, M. et al. Successful tumour necrosis factor (TNF) blocking therapy suppresses oxidative stress and hypoxia-induced mitochondrial mutagenesis in inflammatory arthritis. Arthritis Res. Ther. 13, R121 (2011).
Garcia-Ruiz, I. et al. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology 44, 581–591 (2006).
Heller, S. et al. Reduced mitochondrial activity in colonocytes facilitates AMPKalpha2-dependent inflammation. FASEB J. 31, 2013–2025 (2017).
Marchini, T. et al. Selective TNF-alpha targeting with infliximab attenuates impaired oxygen metabolism and contractile function induced by an acute exposure to air particulate matter. Am. J. Physiol. Heart Circ. Physiol. 309, H1621–H1628 (2015).
Billmeier, U., Dieterich, W., Neurath, M. F. & Atreya, R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J. Gastroenterol. 22, 9300–9313, (2016).
Wang, A. et al. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am. J. Pathol. 184, 2516–2527 (2014).
Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 13, 7210–7226 (2008).
Merkwirth, C. et al. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 22, 476–488 (2008).
Nijtmans, L. G. et al. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 19, 2444–2451 (2000).
Adolph, T. E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013).
Yang, M. et al. Mitochondria-associated ER membranes — the origin site of autophagy. Front. Cell Dev. Biol. 8, 595 (2020).
Grey, M. J. et al. The epithelial-specific ER stress sensor ERN2/IRE1beta enables host–microbiota crosstalk to affect colon goblet cell development. J. Clin. Invest. 132, e153519 (2022).
Hasnain, S. Z. et al. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 144, 357–368.e9 (2013).
Naama, M. et al. Autophagy controls mucus secretion from intestinal goblet cells by alleviating ER stress. Cell Host Microbe 31, 433–446.e4 (2023).
Zhao, Z. et al. Bisphenol A inhibits mucin 2 secretion in intestinal goblet cells through mitochondrial dysfunction and oxidative stress. Biomed. Pharmacother. 111, 901–908 (2019).
Wei, J. et al. Upregulation of RIP3 promotes necroptosis via a ROS-dependent NF-kappaB pathway to induce chronic inflammation in HK-2 cells. Mol. Med. Rep. 24, 783 (2021).
Yang, Z. et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat. Cell Biol. 20, 186–197 (2018).
Patankar, J. V. & Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 17, 543–556 (2020).
Dhuriya, Y. K. & Sharma, D. Necroptosis: a regulated inflammatory mode of cell death. J. Neuroinflammation 15, 199 (2018).
Prochnicki, T. & Latz, E. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab. 26, 71–93 (2017).
Kummer, J. A. et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem. Cytochem. 55, 443–452 (2007).
Zhen, Y. & Zhang, H. NLRP3 inflammasome and inflammatory bowel disease. Front. Immunol. 10, 276 (2019).
Yu, P. et al. Pyroptosis: mechanisms and diseases. Signal. Transduct. Target. Ther. 6, 128 (2021).
Weindel, C. G. et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell 185, 3214–3231.e23 (2022).
Bauer, C. et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59, 1192–1199 (2010).
Liu, L. et al. The pathogenic role of NLRP3 inflammasome activation in inflammatory bowel diseases of both mice and humans. J. Crohns Colitis 11, 737–750 (2017).
Dashdorj, A. et al. Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 11, 178 (2013).
Allen, I. C. et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207, 1045–1056 (2010).
Dupaul-Chicoine, J. et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32, 367–378 (2010).
Hirota, S. A. et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm. Bowel Dis. 17, 1359–1372 (2011).
Lebeis, S. L., Powell, K. R., Merlin, D., Sherman, M. A. & Kalman, D. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 77, 604–614 (2009).
Takagi, H. et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand. J. Gastroenterol. 38, 837–844 (2003).
Zaki, M. H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Nowarski, R. et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163, 1444–1456 (2015).
Boyapati, R. K. et al. Mitochondrial DNA is a pro-inflammatory damage-associated molecular pattern released during active IBD. Inflamm. Bowel Dis. 24, 2113–2122 (2018).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Zhong, Z. et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203 (2018).
Vrablicova, Z. et al. Nuclear and mitochondrial circulating cell-free DNA is increased in patients with inflammatory bowel disease in clinical remission. Front. Med. 7, 593316 (2020).
Boyapati, R. et al. Mitochondrial DNA is a pro-inflammatory damage-associated molecular pattern (DAMP) released during active IBD. Inflamm. Bowel Dis. 24, 2113–2113 (2018).
Kim, J. et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 366, 1531–1536 (2019).
Verma, A. et al. The role of the mitochondrial protein VDAC1 in inflammatory bowel disease: a potential therapeutic target. Mol. Ther. 30, 726–744 (2022).
Chen, C., Yan, W., Tao, M. & Fu, Y. NAD(+) metabolism and immune regulation: new approaches to inflammatory bowel disease therapies. Antioxidants 12, 1230 (2023).
Kang, Y. H. et al. Metabolic analyses reveal dysregulated NAD+ metabolism and altered mitochondrial state in ulcerative colitis. PLoS ONE 17, e0273080 (2022).
Novak, E. A. et al. Epithelial NAD(+) depletion drives mitochondrial dysfunction and contributes to intestinal inflammation. Front. Immunol. 14, 1231700 (2023).
Xue, X., Miao, Y. & Wei, Z. Nicotinamide adenine dinucleotide metabolism: driving or counterbalancing inflammatory bowel disease? FEBS Lett. 597, 1179–1192 (2023).
Fernandez-Marcos, P. J. & Auwerx, J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93, 884S–890S (2011).
Ji, Z., Liu, G. H. & Qu, J. Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genomics 49, 287–298 (2022).
Igarashi, M. & Guarente, L. mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 166, 436–450 (2016).
Gerner, R. R. et al. NAD metabolism fuels human and mouse intestinal inflammation. Gut 67, 1813–1823 (2018).
Lv, Q. et al. Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD(+)/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death Dis. 9, 258 (2018).
Furuta, G. T. et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 193, 1027–1034 (2001).
Colgan, S. P., Wang, R. X., Hall, C. H. T., Bhagavatula, G. & Lee, J. S. Revisiting the ‘starved gut’ hypothesis in inflammatory bowel disease. Immunometabolism 5, e0016 (2023).
Andoh, A. Physiological role of gut microbiota for maintaining human health. Digestion 93, 176–181 (2016).
Eckburg, P. B. et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005).
Sheehan, D., Moran, C. & Shanahan, F. The microbiota in inflammatory bowel disease. J. Gastroenterol. 50, 495–507 (2015).
den Besten, G. et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340 (2013).
Morrison, D. J. & Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200 (2016).
Derrien, M., Vaughan, E. E., Plugge, C. M. & de Vos, W. M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 54, 1469–1476 (2004).
Joyce, S. A. & Gahan, C. G. Bile acid modifications at the microbe–host interface: potential for nutraceutical and pharmaceutical interventions in host health. Annu. Rev. Food Sci. Technol. 7, 313–333 (2016).
Macfarlane, S. & Macfarlane, G. T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 62, 67–72 (2003).
Ridlon, J. M., Kang, D. J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259 (2006).
Hofmann, A. F. Bile acids: the good, the bad, and the ugly. N. Physiol. Sci. 14, 24–29 (1999).
Pavlidis, P. et al. Systematic review: bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence? Aliment. Pharmacol. Ther. 42, 802–817 (2015).
Litvak, Y. & Baumler, A. J. Microbiota-nourishing immunity: a guide to understanding our microbial self. Immunity 51, 214–224 (2019).
Litvak, Y., Byndloss, M. X. & Baumler, A. J. Colonocyte metabolism shapes the gut microbiota. Science 362, eaat9076 (2018).
Tiffany, C. R. & Baumler, A. J. Dysbiosis: from fiction to function. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G602–G608 (2019).
Pena-Cearra, A. et al. Mitochondrial dysfunction promotes microbial composition that negatively impacts on ulcerative colitis development and progression. npj Biofilms Microbiomes 9, 74 (2023).
Rath, E. & Haller, D. Intestinal epithelial cell metabolism at the interface of microbial dysbiosis and tissue injury. Mucosal Immunol. 15, 595–604 (2022).
Deleu, S., Machiels, K., Raes, J., Verbeke, K. & Vermeire, S. Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? eBioMedicine 66, 103293 (2021).
Litvak, Y., Byndloss, M. X., Tsolis, R. M. & Baumler, A. J. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 39, 1–6 (2017).
Rivera-Chavez, F., Lopez, C. A. & Baumler, A. J. Oxygen as a driver of gut dysbiosis. Free Radic. Biol. Med. 105, 93–101 (2017).
Ray, K. Mitochondrial dysfunction in Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 17, 260 (2020).
Alula, K. M. et al. Interplay of gut microbiota and host epithelial mitochondrial dysfunction is necessary for the development of spontaneous intestinal inflammation in mice. Microbiome 11, 256 (2023).
Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019).
Miyoshi, H. et al. Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. EMBO J. 36, 5–24 (2017).
Quiros, M. & Nusrat, A. Contribution of wound-associated cells and mediators in orchestrating gastrointestinal mucosal wound repair. Annu. Rev. Physiol. 81, 189–209 (2019).
Eastwood, G. L. Gastrointestinal epithelial renewal. Gastroenterology 72, 962–975 (1977).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Yui, S. et al. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22, 35–49.e7 (2018).
Berger, E. et al. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat. Commun. 7, 13171 (2016).
Mancini, N. L. et al. Perturbed mitochondrial dynamics is a novel feature of colitis that can be targeted to lessen disease. Cell. Mol. Gastroenterol. Hepatol. 10, 287–307 (2020).
Youle, R. J. & van der Bliek, A. M. Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 (2012).
Bhogoju, S. et al. A novel drug therapy induces mitochondrial biogenesis and attenuates colitis. Inflamm. Bowel Dis. 29, S55–S55 (2023).
Paladines, N. et al. Metabolic reprogramming through mitochondrial biogenesis drives adenosine anti-inflammatory effects: new mechanism controlling gingival fibroblast hyper-inflammatory state. Front. Immunol. 14, 1148216 (2023).
Thankam, F. G. et al. Amplification of mitochondrial activity in the healing response following rotator cuff tendon injury. Sci. Rep. 8, 17027 (2018).
Willenborg, S. et al. Mitochondrial metabolism coordinates stage-specific repair processes in macrophages during wound healing. Cell Metab. 33, 2398–2414.e9 (2021).
Ussakli, C. H. et al. Mitochondria and tumor progression in ulcerative colitis. J. Natl Cancer Inst. 105, 1239–1248 (2013).
Onishi, M., Yamano, K., Sato, M., Matsuda, N. & Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 40, e104705 (2021).
Foerster, E. G. et al. How autophagy controls the intestinal epithelial barrier. Autophagy 18, 86–103 (2022).
Nguyen, H. T., Lapaquette, P., Bringer, M. A. & Darfeuille-Michaud, A. Autophagy and Crohn’s disease. J. Innate Immun. 5, 434–443 (2013).
Matsuzawa-Ishimoto, Y. et al. Autophagy protein ATG16L1 prevents necroptosis in the intestinal epithelium. J. Exp. Med. 214, 3687–3705 (2017).
Vincent, G. et al. Nix-mediated mitophagy modulates mitochondrial damage during intestinal inflammation. Antioxid. Redox Signal. 33, 1–19 (2020).
Alula, K. M. et al. Inner mitochondrial membrane protein Prohibitin 1 mediates Nix-induced, Parkin-independent mitophagy. Sci. Rep. 13, 18 (2023).
Centelles, J. J. General aspects of colorectal cancer. ISRN Oncol. 2012, 139268 (2012).
Morgan, E. et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72, 338–344 (2023).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Schwitalla, S. et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152, 25–38 (2013).
Shih, I.-M. et al. Top-down morphogenesis of colorectal tumors. Proc. Natl Acad. Sci. USA 98, 2640–2645 (2001).
Kasi, A. et al. Molecular pathogenesis and classification of colorectal carcinoma. Curr. Colorectal Cancer Rep. 16, 97–106 (2020).
Baker, A.-M. et al. Evolutionary history of human colitis-associated colorectal cancer. Gut 68, 985–995 (2019).
Warburg, O. The Metabolism of Tumours (Constable, 1930).
Li, W. et al. Comprehensive analysis of the association between tumor glycolysis and immune/inflammation function in breast cancer. J. Transl. Med. 18, 92 (2020).
Nantasupha, C., Thonusin, C., Charoenkwan, K., Chattipakorn, S. & Chattipakorn, N. Metabolic reprogramming in epithelial ovarian cancer. Am. J. Transl. Res. 13, 9950–9973 (2021).
Cui, J. et al. FOXM1 promotes the Warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin. Cancer Res. 20, 2595–2606 (2014).
Al-Khayal, K. et al. Identification of the TP53-induced glycolysis and apoptosis regulator in various stages of colorectal cancer patients. Oncol. Rep. 35, 1281–1286 (2016).
Graziano, F. et al. Glycolysis gene expression analysis and selective metabolic advantage in the clinical progression of colorectal cancer. Pharmacogenomics J. 17, 258–264 (2017).
Chance, B. & Hess, B. On the control of metabolism in ascites tumor cell suspensions. Ann. N. Y. Acad. Sci. 63, 1008–1016 (1956).
Burk, D. & Schade, A. L. On respiratory impairment in cancer cells. Science 124, 270–272 (1956).
Lunt, S. Y. & Heiden, M. G. V. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011).
Kirsch, M. & De Groot, H. NAD(P)H, a directly operating antioxidant? FASEB J. 15, 1569–1574 (2001).
Pfeiffer, T., Schuster, S. & Bonhoeffer, S. Cooperation and competition in the evolution of ATP-producing pathways. Science 292, 504–507 (2001).
Vaupel, P., Schmidberger, H. & Mayer, A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 95, 912–919 (2019).
Mana, M. D. et al. High-fat diet-activated fatty acid oxidation mediates intestinal stemness and tumorigenicity. Cell Rep. 35, 109212 (2021).
Zhao, Y. et al. Colorectal cancers utilize glutamine as an anaplerotic substrate of the TCA cycle in vivo. Sci. Rep. 9, 19180 (2019).
Taylor, R. et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J. Clin. Investig. 112, 1351–1360 (2003).
Greaves, L. C. et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc. Natl Acad. Sci. USA 103, 714–719 (2006).
Ju, Y. S. et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. eLife 3, e02935 (2014).
Guo, W. et al. Mutational signature of mtDNA confers mechanistic insight into oxidative metabolism remodeling in colorectal cancer. Theranostics 13, 324–338 (2023).
Smith, A. L. et al. Age-associated mitochondrial DNA mutations cause metabolic remodelling that contributes to accelerated intestinal tumorigenesis. Nat. Cancer 1, 976–989 (2020).
Mou, J.-j et al. Mitochondrial DNA content reduction induces aerobic glycolysis and reversible resistance to drug-induced apoptosis in SW480 colorectal cancer cells. Biomed. Pharmacother. 103, 729–737 (2018).
Bensard, C. L. et al. Regulation of tumor initiation by the mitochondrial pyruvate carrier. Cell Metab. 31, 284–300.e7 (2020).
Schell, J. C. et al. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol. Cell 56, 400–413 (2014).
Darvey, I. G. What factors are responsible for the greater yield of ATP per carbon atom when fatty acids are completely oxidised to CO2 and water compared with glucose? Biochem. Educ. 27, 209–210 (1999).
Lin, C.-S. et al. Role of mitochondrial function in the invasiveness of human colon cancer cells. Oncol. Rep. 39, 316–330 (2018).
Sun, X. et al. Increased mtDNA copy number promotes cancer progression by enhancing mitochondrial oxidative phosphorylation in microsatellite-stable colorectal cancer. Signal. Transduct. Target. Ther. 3, 8 (2018).
Rebane-Klemm, E. et al. Mitochondrial respiration in KRAS and BRAF mutated colorectal tumors and polyps. Cancers 12, 815 (2020).
Hernández-López, R. et al. Mitochondrial function differences between tumor tissue of human metastatic and premetastatic CRC. Biology 11, 293 (2022).
Wen, Y. A. et al. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 8, e2593 (2017).
Wang, Y.-n et al. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene 37, 6025–6040 (2018).
Devenport, S. N. et al. Colorectal cancer cells utilize autophagy to maintain mitochondrial metabolism for cell proliferation under nutrient stress. JCI Insight 6, e138835 (2021).
Wise, D. R. & Thompson, C. B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 35, 427–433 (2010).
Wang, T. et al. MTA1, a novel ATP synthase complex modulator, enhances colon cancer liver metastasis by driving mitochondrial metabolism reprogramming. Adv. Sci. 10, 2300756 (2023).
Song, I.-S. et al. FOXM1-induced PRX3 regulates stemness and survival of colon cancer cells via maintenance of mitochondrial function. Gastroenterology 149, 1006–1016.e9 (2015).
Denise, C. et al. 5-Fluorouracil resistant colon cancer cells are addicted to OxPHOS to survive and enhance stem-like traits. Oncotarget 6, 41706–41721 (2015).
Yun, C. W., Han, Y.-S. & Lee, S. H. PGC-1α controls mitochondrial biogenesis in drug-resistant colorectal cancer cells by regulating endoplasmic reticulum stress. Int. J. Mol. Sci. 20, 1707 (2019).
Disoma, C., Zhou, Y., Li, S., Peng, J. & Xia, Z. Wnt/β-catenin signaling in colorectal cancer: is therapeutic targeting even possible? Biochimie 195, 39–53 (2022).
Bernkopf, D. B. et al. Pgam5 released from damaged mitochondria induces mitochondrial biogenesis via Wnt signaling. J. Cell Biol. 217, 1383–1394 (2018).
Zhang, W. et al. An underlying mechanism of dual Wnt inhibition and AMPK activation: mitochondrial uncouplers masquerading as Wnt inhibitors. J. Med. Chem. 62, 11348–11358 (2019).
Costa, R. et al. Impaired mitochondrial ATP production downregulates Wnt signaling via ER stress induction. Cell Rep. 28, 1949–1960.e6 (2019).
Wen, Y.-A. et al. The mitochondrial retrograde signaling regulates Wnt signaling to promote tumorigenesis in colon cancer. Cell Death Differ. 26, 1955–1969 (2019).
Hu, J. L. et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial–mesenchymal transition in colorectal cancer. Mol. Cancer 18, 91 (2019).
Pate, K. T. et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 33, 1454–1473 (2014).
Xiong, X. et al. Activation of Drp1 promotes fatty acids-induced metabolic reprograming to potentiate Wnt signaling in colon cancer. Cell Death Differ. 29, 1913–1927 (2022).
Suman, S., Das, T. P., Ankem, M. K. & Damodaran, C. Targeting notch signaling in colorectal cancer. Curr. Colorectal Cancer Rep. 10, 411–416 (2014).
Perumalsamy, L. R., Nagala, M. & Sarin, A. Notch-activated signaling cascade interacts with mitochondrial remodeling proteins to regulate cell survival. Proc. Natl Acad. Sci. USA 107, 6882–6887 (2010).
Gopalakrishnan, N., Sivasithamparam, N. D. & Devaraj, H. Synergistic association of Notch and NFκB signaling and role of Notch signaling in modulating epithelial to mesenchymal transition in colorectal adenocarcinoma. Biochimie 107, 310–318 (2014).
Chen, B. et al. PTEN-induced kinase PINK1 supports colorectal cancer growth by regulating the labile iron pool. J. Biol. Chem. 299, 104691 (2023).
Huang, C.-Y. et al. HMGB1 promotes ERK-mediated mitochondrial Drp1 phosphorylation for chemoresistance through RAGE in colorectal cancer. Cell Death Dis. 9, 1004 (2018).
Jiang, T. et al. Cirsiliol regulates mitophagy in colon cancer cells via STAT3 signaling. Cancer Cell Int. 22, 304 (2022).
Chen, W. T., Yang, H. B., Ke, T. W., Liao, W. L. & Hung, S. Y. Serum DJ-1 is a biomarker of colorectal cancer and DJ-1 activates mitophagy to promote colorectal cancer progression. Cancers 13, 4151 (2021).
Gao, H. et al. DJ-1 protects dopaminergic neurons against rotenone-induced apoptosis by enhancing ERK-dependent mitophagy. J. Mol. Biol. 423, 232–248 (2012).
Ziegler, P. K. et al. Mitophagy in intestinal epithelial cells triggers adaptive immunity during tumorigenesis. Cell 174, 88–101.e16 (2018).
Boyle, K. A. et al. Mitochondria-targeted drugs stimulate mitophagy and abrogate colon cancer cell proliferation. J. Biol. Chem. 293, 14891–14904 (2018).
D’Onofrio, N. et al. Colorectal cancer apoptosis induced by dietary δ-valerobetaine involves PINK1/parkin dependent-mitophagy and SIRT3. Int. J. Mol. Sci. 22, 8117 (2021).
Liang, L. et al. Oxymatrine suppresses colorectal cancer progression by inhibiting NLRP3 inflammasome activation through mitophagy induction in vitro and in vivo. Phytother. Res. 37, 3342–3362 (2023).
Zhang, X. et al. Targeting mitochondrial dysfunction in neurodegenerative diseases: expanding the therapeutic approaches by plant-derived natural products. Pharmaceuticals 16, 277 (2023).
Rousseaux, C. et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J. Exp. Med. 201, 1205–1215 (2005).
Chen, Q. L., Yin, H. R., He, Q. Y. & Wang, Y. Targeting the NLRP3 inflammasome as new therapeutic avenue for inflammatory bowel disease. Biomed. Pharmacother. 138, 111442 (2021).
Lewis, J. D. et al. Rosiglitazone for active ulcerative colitis: a randomized placebo-controlled trial. Gastroenterology 134, 688–695 (2008).
Yao, Q. et al. 2′-Fucosyllactose ameliorates inflammatory bowel disease by modulating gut microbiota and promoting MUC2 expression. Front. Nutr. 9, 822020 (2022).
Mezoff, E. A. et al. The human milk oligosaccharide 2′-fucosyllactose augments the adaptive response to extensive intestinal. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G427–G438 (2016).
Tseng, C. H. Metformin use is associated with a lower risk of inflammatory bowel disease in patients with type 2 diabetes mellitus. J. Crohns Colitis 15, 64–73 (2021).
Wheaton, W. W. et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3, e02242 (2014).
Din, F. V. N. et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 142, 1504–1515.e3 (2012).
Hu, F. et al. Penicillin disrupts mitochondrial function and induces autophagy in colorectal cancer cell lines. Oncol. Lett. 22, 691 (2021).
Schwab, M. et al. PPARgamma is involved in mesalazine-mediated induction of apoptosis and inhibition of cell growth in colon cancer cells. Carcinogenesis 29, 1407–1414 (2008).
Arnold, C. et al. The mitochondrial disruptor devimistat (CPI-613) synergizes with genotoxic anticancer drugs in colorectal cancer therapy in a Bim-dependent manner. Mol. Cancer Ther. 21, 100–112 (2022).
Tomimoto, A. et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 99, 2136–2141 (2008).
Hosono, K. et al. Metformin suppresses azoxymethane‐induced colorectal aberrant crypt foci by activating AMP‐activated protein kinase. Mol. Carcinog. 49, 662–671 (2010).
Zimmermann, K. C., Waterhouse, N. J., Goldstein, J. C., Schuler, M. & Green, D. R. Aspirin induces apoptosis through release of cytochrome c from mitochondria. Neoplasia 2, 505–513 (2000).
Corona, J. C. & Duchen, M. R. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic. Biol. Med. 100, 153–163 (2016).
Chen, Q., Ruan, D., Shi, J., Du, D. & Bian, C. The multifaceted roles of natural products in mitochondrial dysfunction. Front. Pharmacol. 14, 1093038 (2023).
Vatn, S. S. et al. Mucosal gene transcript signatures in treatment naive inflammatory bowel disease: a comparative analysis of disease to symptomatic and healthy controls in the European IBD-Character Cohort. Clin. Exp. Gastroenterol. 15, 5–25 (2022).
Yu, Y. R. & Rodriguez, J. R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 26, 349–355 (2017).
Acknowledgements
This work was supported by the National Institutes of Health R01-DK117001 (A.L.T.), National Institutes of Health R01-DK095662 (T.A.B.), Crohn’s Colitis Foundation SRA 900820 (A.L.T.), Crohn’s and Colitis Foundation, Litwin Award 993820 (T.A.B.), Veteran’s Affairs BLRD MERIT IBX005288 (A.L.T.), Veteran’s Affairs CSRD MERIT 1I01CX001353 (T.A.B.) and GI & Liver Innate Immune Program (GALIIP) — University of Colorado Anschutz (A.L.T.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. A.L.T. and T.A.B. contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Lee Denson, Dirk Haller, Kevin Mollen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
NCT00065065: https://clinicaltrials.gov/study/NCT00065065
NCT00790543: https://clinicaltrials.gov/study/NCT00790543
NCT01536535: https://clinicaltrials.gov/study/NCT01536535
NCT02232152: https://clinicaltrials.gov/study/NCT02232152
NCT04276740: https://clinicaltrials.gov/study/NCT04276740
NCT05539625: https://clinicaltrials.gov/study/NCT05539625
NCT05561062: https://clinicaltrials.gov/study/NCT05561062
NCT05561738: https://clinicaltrials.gov/study/NCT05561738
NCT05567068: https://clinicaltrials.gov/study/NCT05567068
NCT05753267: https://clinicaltrials.gov/study/NCT05753267
NCT05781698: https://clinicaltrials.gov/study/NCT05781698
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haque, P.S., Kapur, N., Barrett, T.A. et al. Mitochondrial function and gastrointestinal diseases. Nat Rev Gastroenterol Hepatol (2024). https://doi.org/10.1038/s41575-024-00931-2
Accepted:
Published:
DOI: https://doi.org/10.1038/s41575-024-00931-2